
MEDICAL DEVICE MATERIAL PERFORMANCE STUDY
Hyaluronic Acid (HA)
Safety Profile
Report Details
Date of Submission
December 5, 2021
Prepared For
U.S. FDA Center for Devices and Radiological Health
Submitted to
Ed Margerrison, PhD
Director, Office of Science and Engineering Laboratories (OSEL)
Center for Devices and Radiological Health
U.S. Food and Drug Administration
ECRI Corporate Governance
Michael Argentieri, MS
Vice President, Technology and Safety
Phone: (610) 316-2766
Email: [email protected]
Dheerendra Kommala, MD
Chief Medical Officer, Executive Committee
Phone: (610) 825-6000 ext. 5090
Email: dkommala@ECRI.org
Project Manager
Christopher N. Schabowsky, PhD, CCE
Director, Accident and Forensic Investigation
Phone: (540) 272-2740
Email: cschabowsky@ECRI.org
If you have any questions or require additional information, please do not hesitate to call the Project Manager.

Material Performance Study - Hyaluronic Acid
|
2
Table of Contents
Introduction ..................................................................................................................................................................... 4
Executive Summary – Muscle/Skeletal Applications ............................................................................................................. 5
Executive Summary – Dermal, Facial and Eye Applications ................................................................................................ 10
Executive Summary – Adhesion Barrier and Bulking Agent Applications .............................................................................. 13
Project Overview ............................................................................................................................................................. 16
Literature Search and Systematic Review Framework ........................................................................................................ 17
ECRI Surveillance Search Strategy ................................................................................................................................... 18
Safety Profile – Muscle/Skeletal Applications ..................................................................................................................... 20
Safety Brief - Systematic Review Results ........................................................................................................................ 20
ECRI Surveillance Data .................................................................................................................................................. 29
Potential Gaps .............................................................................................................................................................. 31
Safety Profile – Dermal, Facial and Eye Applications .......................................................................................................... 33
Safety Brief - Systematic Review Results ........................................................................................................................ 33
ECRI Surveillance Data .................................................................................................................................................. 40
Potential Gaps .............................................................................................................................................................. 41
Safety Profile – Adhesion Barrier and Bulking Agent Applications........................................................................................ 43
Safety Brief - Systematic Review Results ........................................................................................................................ 43
ECRI Surveillance Data .................................................................................................................................................. 52
Potential Gaps .............................................................................................................................................................. 53
Appendix A. Inclusion/Exclusion Criteria and Quality of Evidence Criteria ............................................................................ 54
Appendix B1. Search Summary – Muscle/Skeletal Applications ........................................................................................... 55
Appendix B2. Search Summary – Dermal, Facial and Eye Applications ................................................................................ 58
Appendix B3. Search Summary – Adhesion Barrier and Bulking Agent Applications .............................................................. 61
Appendix C1. Study Flow Diagram – Muscle/Skeletal Applications ...................................................................................... 68
Appendix C2. Study Flow Diagram – Dermal, Facial and Eye Applications ........................................................................... 69
Appendix C3. Study Flow Diagram – Adhesion Barrier and Bulking Agent Applications ......................................................... 69
Appendix D1. Evidence Tables – Muscle/Skeletal Applications ............................................................................................ 71
Appendix D2. Evidence Tables – Dermal, Facial and Eye Applications .................................... Error! Bookmark not defined.
Appendix D3. Evidence Tables – Adhesion Barrier and Bulking Agent Applications .............................................................. 130
Appendix E. References .................................................................................................................................................. 166
Appendix F. Surveillance Event Reports - PSO and Accident Investigation .......................................................................... 176

Material Performance Study - Hyaluronic Acid
|
3
Appendix G. Regulatory and Manufacturer Safety Alerts ................................................................................................... 177
Table of Tables
Table 1: Medical Devices Containing HA for Muscle/Skeletal Applications provided by FDA to Guide ECRI Searches.............. 20
Table 2: Summary of Primary Findings from Systematic Review – Muscle/Skeletal Applications ........................................... 20
Table 3" Complications in HA-related PSO Event Reports – Muscle/Skeletal Applications ..................................................... 30
Table 4: Harm Score Associated with HA-related PSO Event Reports – Muscle/Skeletal Applications .................................... 30
Table 5: Summary of Regulatory and Manufacturer Alerts - Muscle/Skeletal Applications .................................................... 31
Table 6: Medical Devices Containing HA for Dermal, Facial and Eye Applications provide by FDA to Guide ECRI Searches .... 33
Table 7: Summary of Primary Findings from Systematic Review - Dermal, Facial and Eye Applications ................................ 34
Table 8: Complications in HA-related PSO Event Reports - Dermal, Facial and Eye Applications .......................................... 40
Table 9: Harm Score Associated with HA-related PSO Event Reports - Dermal, Facial, and Eye Applications ........................ 40
Table 10: Summary of Regulatory and Manufacturer Alerts - Dermal, Facial and Eye Applications ....................................... 41
Table 11: Medical Devices Containing HA for Adhesion Barrier and Bulking Agent Applications provided by FDA to Guide ECRI
Searches ........................................................................................................................................................................ 43
Table 12: Summary of Primary Findings from Systematic Review - Adhesion Barrier and Bulking Agent Applications ............ 44
Table 13: Summary of Regulatory and Manufacturer Alerts - Adhesion Barrier and Bulking Agent Applications .................... 53
Table 14: Viscosupplementation – knee: Health Effect (In Vivo) Human Studies ................................................................ 71
Table 15: Viscosupplementation – hip: Health Effect (In Vivo) Human Studies ................................................................... 88
Table 16: Viscosupplementation – hand and ankle: Health Effect (In Vivo) Human Studies ................................................. 90
Table 17: Viscosupplementation – shoulder: Health Effect (In Vivo) Human Studies ........................................................... 92
Table 18: Viscosupplementation – temporomandibular joint disorders: Health Effect (In Vivo) Human Studies ..................... 93
Table 19: Scaffold - Health Effect (In Vivo) Human Studies .............................................................................................. 95
Table 20: Dermal Fillers - Health Effect (In Vivo) Human Studies ...................................................................................... 97
Table 21: Dermal Fillers - Health Effects (In Vivo) Animal Studies .................................................................................... 116
Table 22: Intraocular/Ophthalmic Viscoelastic Solution/Fluid - Health Effect (In Vivo) Human Studies ................................ 117
Table 23: Eye Drops - Health Effect (In Vivo) Human Studies .......................................................................................... 123
Table 24: Hyaluronic acid as a Material – Health Effect (In Vivo) Human Studies .............................................................. 130
Table 25: Adhesion Barrier - Health Effects (In Vivo) Human Studies ............................................................................... 132
Table 26: Anti-adhesion: Nasal packing - Health Effect (In Vivo) Human Studies .............................................................. 151
Table 27: Barrier gel for oral lesions - Health Effect (In Vivo) Human Studies ................................................................... 154
Table 28: Bulking Agents - Health Effect (In Vivo) Human Studies ................................................................................... 156
Table 29: : Intravesical agents for bladder pain syndrome/interstitial cystitis therapy - Health Effect (In Vivo) Human Studies
..................................................................................................................................................................................... 159
Table 30: Organ spacer - Health Effect (In Vivo) Human Studies ..................................................................................... 161
Table 31: Protective topical agent for esophageal and gastric lesions in GERD - Health Effect (In Vivo) Human Studies ...... 163
Table 32: Vocal cord/fold medialization - Health Effect (In Vivo) Human Studies ............................................................... 165

Material Performance Study - Hyaluronic Acid
|
4
Introduction
This Medical Device Materials Performance Report divides hyaluronic acid into three major categories:
1. Muscle/skeletal applications
2. Dermal, facial and eye applications
3. Adhesion barrier and bulking agent applications
Accordingly, the report has dedicated sections and discussions for each application. These include separate executive
summaries, safety briefs, surveillance data, gap analyses, and appendices. The goal of this report structure is to allow the
reader to focus on the current state of knowledge with regard to medical device material biocompatibility for each application
in an organized and discrete manner.

Material Performance Study - Hyaluronic Acid
|
5
Executive Summary – Muscle/Skeletal Applications
Key Points
1. Searches identified 1795 citations; 39 articles were selected for inclusion.
2. Thirty-six (92%) studies in the evidence base focused on viscosupplementation, the intra-articular (IA) injection of
hyaluronic acid (HA). Of these 36 studies, 26 studies addressed knees, 4 studies addressed hips, while 2 studies each
addressed hands or ankles, shoulders, and temporomandibular joint (TMJ) disorders. Three remaining studies
examined HA as cartilage scaffolds.
3. The most commonly reported local responses included swelling, pain at injection site, arthralgia/joint pain, and
effusion which were associated with moderate to low quality of evidence. Additionally, joint stiffness, musculoskeletal
pain, post-injection pain, pain flare ups and edema were frequently reported, and they were associated with low
quality of evidence. Outcomes infrequently reported (e.g., hematoma) or device categories with no evidence (HA as
a material, and bone putty/filler) were rated very low quality of evidence.
4. Evidence for systemic responses was reported for viscosupplementation in knees, shoulders, and TMJ disorders. One
systematic review (SR) investigating fatal adverse events (AEs) from intraarticular injections of HA listed in the
Manufacturer and User Facility Device Experience (MAUDE) and Alternative Summary Reporting databases between
2014 and 2019 identified 63 unique fatalities from knee viscosupplementation. Eight (12%) fatalities were
categorized as possibly related to the intraarticular injection of HA (IAHA), but available information was “insufficient
to make a firm determination of causality.” Two studies reporting skin reactions (1 rash, 1 peeling of skin on hands
and toes) directly attributed the response to knee viscosupplementation.
5. Healthcare Technology Alerts identified 5 manufacturer issued alerts describing problems with adverse events,
contamination, packaging issues, and impartially filled products.
6. Patient Safety Organization identified 7 complications associated with HA including wrong side procedure - 2
(28.5%), 2) wrong product - 2 (28.5%), 3) product expired - 2 (28.5%), and 4) wrong time – 1 (14.2%).
7. Evidence gaps:
a. Additional research is needed in viscosupplementation in non-knee indications. Small evidence bases were
identified for hip, hand, ankle, shoulder and TMJ disorders. While studies for other indications (e.g., plantar
fasciitis, trigger finger, and rotator cuff) were identified, these studies were excluded at final prioritization
level due to being low quality (e.g., single arm design) or having small enrollment (<100 patients).
b. Evidence for viscosupplementation was mostly focused on 2 HA products (Hyalgan and Synvisc). Additional
evidence is needed for Monovisc, HYADD, Sinovial, Gel-One, Supartz, Go-On, Hanox M-XL, Ostenil, and
Hyalubrix which were investigated in ≤3 studies. Additionally, evidence is lacking for numerous HA products
that were not evaluated for viscosupplementation (e.g., Synojoynt, Triluron, Trivisc), bone putty/filler (e.g.,
DBX Putty, Kinex Gel), cartilage scaffold or (e.g., Agili-C).
c. While many studies were high-quality (SRs or randomized controlled trials (RCTs)), several studies did not
report important study characteristics (e.g., HA products investigated, HA dose), or details on clinical results

Material Performance Study - Hyaluronic Acid
|
6
(e.g., type of treatment-related AE (TRAE), number of events, number of patients experiencing events,
timing of events).
d. Long-term human RCTs for local responses to HA as a material and for all device categories to better
ascertain associations with these responses to HA.
e. Additional research on systemic responses, including those on patient or material factors, for all HA device
categories. Systemic responses were only investigated in 13 (33%) studies with no studies investigating HA
for viscosupplementation (in hips, hands, and ankles), bone putty/fillers, or cartilage scaffolds.
Overview - Muscle/Skeletal Applications
FDA engaged ECRI to perform a comprehensive literature search and systematic review to identify the current state of
knowledge with regard to medical device material biocompatibility. Additionally, data derived from ECRI’s Patient Safety
Organization (PSO), accident investigations, Problem Reporting Network (PRN), and healthcare technology alerts were
analyzed. This report focuses on answering five key questions provided by FDA and summarized below, regarding a host’s
local and systemic response to Hyaluronic Acid in muscle/skeletal applications. If data did not exist to sufficiently address
these questions, a gap was noted in this report. These gaps could represent areas of further research.
1. What is the typical/expected local host response to these materials?
Local responses/device events varied somewhat across different device categories and between human studies (see
specific responses/events under 1a. below). The majority of ECRI surveillance data were related to wrong side
application, packaging issues, and contamination, which were not related to material response due to insufficient
biocompatibility or mechanical integrity and use of the device.
a.
Can that response vary by location or type of tissue the device is implanted in or near?
i. Swelling occurred with viscosupplementation in knees and ankles, and scaffold placement in knees.
This local response was the most commonly reported after viscosupplementation in knees. One study
reported mild-to-moderate swelling of the knee joint occurred similarly with HA vs. platelet-rich plasma
(PRP) with HA rates ranging from 87% (6 months) to 100% (6 weeks). Other studies reported higher
but low incidence of knee swelling with IAHA vs. saline (3.7% Synvisc, 0.9% saline; 1.1% Monovisc,
0.5% saline). One RCT reported that swelling after injection of Synvisc was device-related, while 1 SR
of 12 RCTs reported significantly more TRAEs (including joint swelling) occurred with HA vs
corticosteroid (CS; 746 events vs. 606 events). Mild swelling was reported after scaffold placement in
knees (n=3), and also after viscosupplementation in ankles.
ii. Pain at injection site occurred after viscosupplementation in knees, shoulders, ankles, and TMJ
disorders. One study reported knee pain and local swelling at injection site in 21% of patients
administered HA injections of different molecular weights and concentrations (DMW), high molecular
weights (HMWs), and low molecular weights (LMWs). Studies also reported knee pain at injection site
in 8 (13.4%) patients with IAHA, and a 0.5% incidence rate with Synvisc. This outcome was commonly
reported in 1 SR of 15 studies addressing viscosupplementation in shoulders, and in 1 SR of 18 studies
addressing viscosupplementation in ankles. Lastly, 2 SRs examining TMJ disorders reported a lower
incidence of pain at injection site with HA vs. CS (37% vs. 70%) and HA vs. PRP or platelet-rich growth
factor (PRGF; 60% vs. 76%).
iii. Arthralgia/joint pain was reported in 7 studies focused on viscosupplementation in knees. Studies
reported a significantly higher occurrence of arthralgia with HA vs. nonsteroidal anti-inflammatory drugs
(NSAIDs; 8.1% vs. 2.9%), but similar rates vs. saline (<4% in 2 studies). 1 SR reported severe joint
pain in 28 (100%) patients with pseudoseptic arthritis, a rare complication after IAHA injection. 2 RCTs
reported that arthralgia with Synvisc or Monovisc was device-related.
iv. Effusion was reported in 5 studies focused on viscosupplementation in knees. One SR (n=5,354)
reported no significant difference in effusion after injection based on molecular weights (1.9% HMW,

Material Performance Study - Hyaluronic Acid
|
7
1.7% medium molecular weight (MMW), 1.8% LMW), but a significantly higher incidence of effusion
with products processed through extraction of avian-derived molecules (AD-HA) vs. bacterial processes
of biological fermentation (Bio-HA; 3.4% vs. 0.5%). Another SR reported that 28 (100%) patients with
pseudoseptic arthritis presented with effusion.
v. Joint stiffness only occurred with knee viscosupplementation. Studies reported no significant differences
in stiffness with HA vs. PRGF, lower rates with Monovisc vs. saline (0.5% vs. 1.1%), and incidence
rates of 7% with varying weights of IAHA.
vi. Musculoskeletal pain was reported after viscosupplementation in knees and shoulders. For knee
viscosupplementation, 1 study reported more frequent musculoskeletal pain with HA vs. PRP (5% vs.
1.8%). For shoulder viscosupplementation, musculoskeletal pain was commonly reported in 13 (87%)
studies in 1 SR; severe musculoskeletal, a serious adverse event (SAE), was reported in some cases.
vii. Post-injection pain was reported after viscosupplementation in knees and hips. For knee
viscosupplementation, studies reported post-injection pain in 3 patients with IAHA, significantly more
post-injection pain with HA vs. PRP, and lower incidence with Synvisc-One vs. cooled radiofrequency
ablation (CRFA; 3% vs. 8%). For hip viscosupplementation, 1 study reported significantly higher post-
injective pain with PRP vs. HA.
viii. Pain flare ups were reported with viscosupplementation in knees and hips. One SR (n=5,354) reported
significantly more knee flare ups with HA products with HMWs vs. lower molecular weights (13.7%
HMW, 3.3% MMW, 10.7% LMW), and a significantly lower incidence of acute flare ups with AD-HA vs.
Bio-HA (3.0% vs. 13.2%). One RCT reported knee flare up rates of 6% to 36% with Synvisc. Lastly, a
higher occurrence of hip pain flare ups were reported with HA vs. placebo (7% vs. 2.4%).
ix. Edema was reported after knee viscosupplementation and scaffold placement. Injection-site edema and
peripheral edema were only reported with HA vs. placebo (0.5 % vs. 0%) in 1 RCT. Edema or cyst
formation occurred similarly with a Hyalofast-based scaffold vs. a chitosan-glycerol phosphate/blood
implant (BST-CarGel®) (8 (38%) Hyalofast, 10 (40%) BST-CarGel) in 1 nonrandomized comparative
study.
x. Less commonly reported local responses included 1) device-related cutaneous vasculitis, Baker’s cyst
(n=2) with Synvisc-One, and elevated inflammatory cell count (n=16) after knee
viscosupplementation; 2) hematoma after hip viscosupplementation and HA scaffold placement in the
knee; 3) pseudogout after ankle viscosupplementation; 4) abscess after shoulder viscosupplementation;
5) postoperative discomfort and ear pressure after viscosupplementation for TMJ disorders; and 6)
persistent pain after an HA scaffold placement in knees.
xi. The overall quality of evidence related to local host responses was moderate to very low, with variation
across different device categories.
xii. Evidence was limited for HA as a scaffold, however no evidence was included for HA as a material, and
bone putty/filler.
b.
Over what time course does this local host response appear?
Swelling was reported after knee and ankle viscosupplementation, from 2 weeks to 6 weeks with an HA
scaffold; and at 6 weeks, 3 months and 6 months with knee viscosupplementation. Pain at injection site was
reported up to 3 days with viscosupplementation of knees, ankles, shoulders, and TMJ disorders. After knee
viscosupplementation: 1) arthralgia was reported in 7 studies as immediately after up to 9 days; 2) effusion
was reported immediately after up to 3 days; and 3) joint stiffness only occurred immediately after. Pain
flare ups were described at weeks 6, 13, 26, 39, and 52 with Synvisc in knee viscosupplementation, and
during or immediately after with hip viscosupplementation. Timing for musculoskeletal pain
(viscosupplementation in knees, shoulders), post-injection pain (viscosupplementation in knees, hips), and
edema was not reported.
2. Does the material elicit a persistent or exaggerated response that may lead to systemic signs or
symptoms – beyond known direct toxicity problems?
a.
What evidence exists to suggest or support this?

Material Performance Study - Hyaluronic Acid
|
8
Overall, 13 studies investigated systemic responses; studies addressed viscosupplementation in knees (11),
shoulders (1), and TMJ disorders (1). Of studies investigating, 11 (85%) studies identified persistent or
exaggerated immune responses, while 26 (67%) did not investigate systemic responses.
b.
What are the likely systemic manifestations?
For knee viscosupplementation, evidence for systemic responses was provided in studies examining IAHA
alone, and studies grouped in the following categories (vs. PRP, anti-inflammatories, saline/placebo,
miscellaneous treatments).
IAHA
: 1 SR indicated that 8 (12%) of 63 fatalities were possibly IAHA-related but available
information was “insufficient to make a firm determination of causality.” Authors noted the
following responses occurred prior to death: septic shock followed by paralysis, necrotizing fasciitis,
and septicemia. Another SR investigating acute pseudoseptic arthritis reported elevated C reactive
protein (CRP), elevated erythrocyte sedimentation rates (ESR), elevated polymorphonuclear
leukocytes (PMNs) in 10 (37%) patients, fever in 6 (22%) patients, and leukocytosis above 10,000
in 4 (15%) patients. Systemic responses in a related case report included a cell count of 123,260
and white blood cell count (WBC) of 15.5 in 1 patient.
IAHA vs. PRP
: 1 of 2 studies investigating identified nasopharyngitis and backache in 2 patients
each, and headache in 1 patient.
IAHA vs. anti-inflammatories
: Of 3 SRs investigating, 1 SR reported significantly more headache vs.
oral NSAIDs (8.4% vs. 4.4%), and cardiac-related complications (n=1). Data for other responses
(nausea and vomiting) was not provided in another SR. Lastly, 1 SR (n=1794) did not identify any
treatment-related systemic responses.
IAHA vs. saline/placebo
: 1 SR of 9 RCTs (n=1967), focused on TRAEs, reported no significant
difference in system-organ class(SOC)-related AEs with IAHA vs. placebo. Cardiac disorders,
vascular disorders, and renal/urinary disorders occurred in <10 patients each with IAHA.
Skin/subcutaneous tissue disorders and hypersensitivity reactions occurred in 22 and 23 patients,
respectively. Gastrointestinal (GI) disorders, respiratory/thoracic/mediastinal disorders, and
nervous system disorders occurred in 66 to 71 patients each. Musculoskeletal and connective tissue
disorders occurred in 145 patients with HA, and 149 patients with placebo. 1 RCT reported a
Monovisc-related rash in 1 patient. Lastly, 1 SR of 10 RCTs attributed 1 serious AE (skin reaction
characterized by peeling of skin on hands and toes) to IAHA.
IAHA vs. miscellaneous treatments
: GI complaints were reported in 3% to 7% of patients.
For shoulder viscosupplementation: 1 SR with followup up to 3 years, commonly reported systemic
responses including diarrhea, flu symptoms, and headache. Serious AEs such as chest pain and cancer were
also reported (N not reported). Responses were not deemed as device-related.
For viscosupplementation in TMJ disorders: 1 individual RCT in a SR reported slight chills in 5.7% of patients
with HA, vs. 0% with CS.
c.
What is the observed timeline(s) for the systemic manifestations?
Evidence for timing was limited to the following:
For knee viscosupplementation:
IAHA:
death in 1 of 8 patients was reported 4 months post-IAHA injection. 1 SR addressing pseudoseptic
arthritis indicated that most cases presented within 24 hours of injection, however time from injection to
presentation ranged from 1 hour to 9 days. In the related case report, arthrocentesis was undertaken at 20
hours post-injection.
IAHA vs.
saline/placebo:
Skin reaction in 1 patient occurred 8 days post-IAHA injection.
IAHA vs. miscellaneous treatments
: GI complaints were tracked at weeks 6, 13, 26, 39, and 52.

Material Performance Study - Hyaluronic Acid
|
9
For viscosupplementation in TMJ disorders: Slight chills were reported at 1 week.
d.
Have particular cellular/molecular mechanisms been identified for such manifestations?
No studies investigated cellular/molecular mechanisms for systemic responses.
3. Are there any patient-related factors that may predict, increase, or decrease the likelihood and/or
severity of an exaggerated, sustained immunological/systemic response?
No studies investigated patient-related factors that may predict, increase, or decrease the likelihood of an
exaggerated, sustained immunological/systemic response.
4. Are there any material-related factors that may predict, increase, or decrease the likelihood and/or
severity of an exaggerated, sustained immunological/systemic response?
1 SR indicated that the pathophysiological response for pseudoseptic arthritis (identified in 28 patients) may be due
to the accumulation of viscous material (e.g., phagocytised HA). This same review noted that significantly more
reactions occurred in patients receiving more than a single administration. Results indicated that 7 cases (25%)
occurred after 2 injections, 5 cases (17.9%) after 3 injections, and 13 cases (46.4%) after ≥4 injections.
5. What critical information gaps exist and what research is needed to better understand this issue?
All gaps listed here could benefit from future research.
a. Additional research is needed in viscosupplementation in non-knee indications. Small evidence bases were
identified for hip, hand, ankle, shoulder and TMJ disorders. While we did identify studies for other
indications (e.g., plantar fasciitis, trigger finger, and rotator cuff), these studies were excluded at final
prioritization level due to being low quality (e.g., single arm design) or having small enrollment (<100
patients).
b. Evidence for viscosupplementation was mostly focused on 2 HA products (Hyalgan and Synvisc). Additional
evidence is needed for Monovisc, HYADD, Sinovial, Gel-One, Supartz, Go-On, Hanox M-XL, Ostenil, and
Hyalubrix which were investigated in ≤3 studies. Additionally, evidence is lacking for numerous HA products
that were not evaluated for viscosupplementation (e.g., Synojoynt, Triluron, Trivisc), bone putty/filler (e.g.,
DBX Putty, Kinex Gel), and cartilage scaffold (e.g., Agili-C).
c. While many studies were high-quality (SRs or RCTs), several studies did not report important study
characteristics (e.g., HA products investigated, HA dose), or details on clinical results (e.g., type of TRAE,
number of events, number of patients experiencing events, timing of events).
d. Long-term human and animal RCTs for local responses to HA as a material and for all device categories to
better ascertain associations with these responses to HA.
e. Additional research on systemic responses, including those on patient or material factors, for all HA device
categories. Systemic responses were only investigated in 13 (33%) studies with no studies investigating HA
for viscosupplementation (in hips, hands, and ankles), bone putty/fillers, or cartilage scaffolds.

Material Performance Study - Hyaluronic Acid
|
10
Executive Summary – Dermal, Facial and Eye Applications
Key Points
1. Searches identified 1401 citations; 58 articles were selected for inclusion
2. For HA dermal fillers, the most common complications of facial injections included lumpiness, tenderness, swelling,
and bruising. Delayed inflammatory reactions/events occurred in about 1% of patients; in rare instances granulomas
formed that required surgical removal. Most complication rates were similar between different HA fillers, but delayed-
onset nodules had a higher occurrence rate for Juvederm Volbella. Rare but serious events included partial or
complete vision loss following upper face injections, as well as brain infarcts or hemorrhage. The overall quality of
evidence is moderate.
3. For intraocular/ophthalmic viscoelastic HA solutions, intraoperative pressure (IOP) elevation was a common
complication following eye surgery and injection of HA solutions. IOP usually decreased over time, but Healon GV
and Healon5 were associated with lesser elevations and faster decreases in IOP over 1 week compared to other HA
solutions. Corneal and/or macular edema were relatively rare events occurring at similar rates for different HA
solutions. The overall strength of evidence is moderate for IOP elevation and corneal/macular edema, low for other
adverse events.
4. For eye drops, reported adverse events included itching/stinging/irritation/erythema/keratitis, eye pain, eye
disorders/dry eye, hyperemia/hemorrhage, and infection/viral conjunctivitis. Overall, most studies reported no
difference in adverse event rates between HA eye drops and other treatments or controls. The overall quality of
evidence is moderate.
5. Systemic responses. A small number of patients developed late-onset, inflammatory, non-infectious adverse reactions
related to dermal fillers/implants (including HA fillers) that could be totally or partially considered as autoimmune/
inflammatory syndrome induced by adjuvants (ASIA)-related disorders. Reported symptoms of HA cases included
myalgia, arthralgia/arthritis, fatigue, neurologic complaints, cognitive features, fever, Sicca syndrome, skin
manifestations (including facial nodules), and autoimmune disease. The overall strength of evidence is low for
systemic responses.
6. ECRI PSO identified one incident of hemorrhage/hematoma associated with a dermal implant composed of HA. There
were no relevant reports found in ECRI’s accident investigation, or PRN databases related to devices composed of
HA.
7. Evidence gaps:
a. Across all device categories, the overall quality of evidence was low to very low for potential systemic
responses.

Material Performance Study - Hyaluronic Acid
|
11
b. For intraocular/ophthalmic/viscoelastic solution/fluids, other than IOP elevation and corneal/macular edema
(moderate quality of evidence), all identified local host responses (e.g., ocular tension, inflammation) were
of low quality of evidence.
c. There were no studies that addressed any particular cellular or molecular mechanisms for systemic
manifestations.
d. Additionally, no studies addressed patient-related or material-related factors that could predict the likelihood
and/or severity of immunological/systemic responses.
Overview - Dermal, Facial and Eye Applications
FDA engaged ECRI to perform a comprehensive literature search and systematic review to identify the current state of
knowledge with regard to medical device material biocompatibility. Additionally, data derived from ECRI’s Patient Safety
Organization (PSO), accident investigations, Problem Reporting Network (PRN), and healthcare technology alerts were
analyzed. This report focuses on answering five key questions provided by FDA and summarized below, regarding a host’s
local and systemic response to Hyaluronic Acid in muscle/skeletal applications. If data did not exist to sufficiently address
these questions, a gap was noted in this report. These gaps could represent areas of further research.
1. What is the typical/expected local host response to these materials?
Local responses/device events varied somewhat across different device categories (see specific responses/events under 1a.
below).
a.
Can that response vary by location or type of tissue the device is implanted in or near?
i. For HA dermal fillers, the most common complications of facial injections included lumpiness,
tenderness, swelling, and bruising. Most complication rates were similar between different HA fillers,
but delayed-onset nodules had a higher occurrence rate for Juvederm Volbella. Delayed inflammatory
reactions/events occurred in about 1% of patients; in rare instances granulomas formed that required
surgical removal. Rare but serious events included partial or complete vision loss following upper face
injections, as well as brain infarcts or hemorrhage. HA (Macrolane) injection of breasts was associated
with cysts, capsular contraction, early absorption and sometimes removal of product was required.
ii. For intraocular/ophthalmic viscoelastic HA solutions, intraoperative pressure (IOP) elevation was a
common complication following eye surgery and injection of HA solutions. IOP usually decreased over
time, but Healon GV and Healon5 were associated with lesser elevations and faster decreases in IOP
over 1 week compared to other HA solutions. Corneal and/or macular edema were relatively rare events
occurring at similar rates for different HA solutions. One study reported Descemet membrane
detachment (DMD) in 7% of patients following canaloplasty and Healon GV injection (most cases
resolved without further surgery). One study reported 34 cases of toxic anterior segment syndrome
(TASS) after cataract surgery; the source was identified as HA solution derived from rooster comb.
iii. For eye drops, reported adverse events included itching/stinging/irritation/erythema/keratitis, eye pain,
eye disorders/dry eye, hyperemia/hemorrhage, and infection/viral conjunctivitis. Overall, most studies
reported no difference in adverse event rates between HA eye drops and other treatments or controls.
b.
Over what time course does this local host response appear?
i. For dermal fillers, most events occurred within the first 2 weeks; a smaller percentage of patients
experienced delayed-onset inflammatory reactions/events that occurred months or even years after
injection (3 to 10 years for granulomas).
ii. For intraocular HA solutions, IOP tended to peak at 1 day post-surgery and decrease afterward. Other
complications occurred within 6 hours to 3 months post-surgery, most within the first week.

Material Performance Study - Hyaluronic Acid
|
12
iii. For eye drops, adverse responses occurred within the first 3 months, most within the first month.
2. Does the material elicit a persistent or exaggerated response that may lead to systemic signs or
symptoms – beyond known direct toxicity problems?
a.
What evidence exists to suggest or support this?
Two retrospective single-arm case series reported on cases of patients suffering from late-onset,
inflammatory, non-infectious adverse reactions related to dermal fillers/implants that could be totally or
partially considered as autoimmune/ inflammatory syndrome induced by adjuvants (ASIA)-related disorders.
b.
What are the likely systemic manifestations?
Reported symptoms of HA cases in the larger study included 4 myalgia, 6 arthralgia/arthritis, 6 fatigue, 2
neurologic complaints, 2 cognitive features, 1 fever, 1 Sicca syndrome, 7 skin manifestations (3 facial
nodules), 3 evolvements into autoimmune disease.
c.
What is the observed timeline(s) for the systemic manifestations?
The symptoms occurred between 6- and 317-months following exposure to dermal filler.
d.
Have particular cellular/molecular mechanisms been identified for such manifestations?
The search did not identify any studies that addressed this question.
3. Are there any patient-related factors that may predict, increase, or decrease the likelihood and/or
severity of an exaggerated, sustained immunological/systemic response?
The number of cases related to HA was low and analyzed with cases who had received other dermal fillers, so the
evidence was unclear concerning patient-related factors that predict sustained systemic response.
4. Are there any material-related factors that may predict, increase, or decrease the likelihood and/or
severity of an exaggerated, sustained immunological/systemic response?
The number of cases related to HA was low and analyzed with cases who had received other dermal fillers, so the
evidence was unclear concerning material-related factors that predict sustained systemic response.
5. What critical information gaps exist and what research is needed to better understand this issue?
All gaps listed here could benefit from future research.
a. Across all device categories, the overall quality of evidence was low to very low for potential systemic responses.
b. For intraocular/ophthalmic/viscoelastic solution/fluids, other than IOP elevation and corneal/macular edema
(moderate quality of evidence), all identified local host responses (e.g., ocular tension, inflammation) were of
low quality of evidence.
c. There were no studies that addressed any particular cellular or molecular mechanisms for systemic
manifestations.
d. Additionally, no studies adequately addressed patient-related or material-related factors that could predict the
likelihood and/or severity of immunological/systemic responses.

Material Performance Study - Hyaluronic Acid
|
13
Executive Summary – Adhesion Barrier and Bulking Agent
Applications
Key Points
1. Searches identified 1217 citations; 52 articles were selected for inclusion
2. Adhesion barriers was the most studied application of hyaluronic acid (34 human studies). There are few adverse
events or complications caused by HA-containing products, and the quality of evidence is High (for both local and
systemic responses). One trial found statistically significantly higher adverse event rates with HA adhesion barriers in
the context of colorectal or small bowel surgery while another trial involving gastrointestinal surgery also found
higher adverse event rates with HA adhesion barriers. It is possible that the powder formulation of HA adhesion
barriers was a contributing factor to increased frequency of adverse events due to the potential for greater diffusion
and migration away from the application site to anastomoses
3. For nasal packaging as well as barrier gel for oral lesions, there are few adverse events or complications caused by
HA-containing products. For both of these categories, the evidence was Moderate for local responses and Very Low
for systemic responses. .
4. In two trials of bulking agents, statistically significantly higher adverse event rates with HA were found in the context
of treatment for fecal incontinence or urinary incontinence which occurred within 12 months of surgery.
5. There were no relevant reports found in ECRI’s PSO, accident investigation, or PRN databases related to adhesion
barriers composed of HA. Healthcare Technology Alerts search returned 4 manufacturer issued alerts describing
problems with compromised sterility and off label use.
6. Evidence gaps:
a. For 7 of 9 device categories, no local adverse events were statistically significantly more likely in patients
receiving HA devices compared to patients receiving non-HA devices. This is predominantly because of a
lack of studies with strong design that investigated these events. The exceptions were adhesion barrier and
bulking agents.

Material Performance Study - Hyaluronic Acid
|
14
b. For 7 of 9 device categories, no systemic adverse events were statistically significantly more likely in
patients receiving HA device compared to patients received non-HA devices. This is predominantly because
of a lack of studies with strong design that investigated these events. The exceptions were adhesion barrier
and bulking agents.
c. Only one study related to HA as a material was identified. However, because treated patients received HA
as well as chondroitin sulfate, one cannot determine whether the reported adverse events were due to HA
or chondroitin sulfate or other factors
d. For 8 of 9 device categories, only study involving adhesion barriers investigated the cellular or molecular
mechanisms for systemic manifestations.
Overview - Adhesion Barrier and Bulking Agent Applications
FDA engaged ECRI to perform a comprehensive literature search and systematic review to identify the current state of
knowledge with regard to medical device material biocompatibility. Additionally, data derived from ECRI’s Patient Safety
Organization (PSO), accident investigations, Problem Reporting Network (PRN), and healthcare technology alerts were
analyzed. This report focuses on answering five key questions provided by FDA and summarized below, regarding a host’s
local and systemic response to Hyaluronic Acid in Adhesion Barrier and Bulking Agent applications. If data did not exist to
sufficiently address these questions, a gap was noted in this report. These gaps could represent areas of further research.
1. What is the typical/expected local host response to these materials?
For 7 of 9 device categories, no local adverse events were statistically significantly more likely in patients receiving HA
devices compared to patients receiving non-HA devices. The exceptions were adhesion barrier and bulking agents.
a.
Can that response vary by location or type of tissue the device is implanted in or near?
i. In two trials of adhesion barriers, statistically significantly higher adverse event rates with HA were
found in the context of colorectal or small bowel surgery (one trial), and “gastrointestinal surgery” (the
other trial. In two trials of bulking agents, statistically significantly higher adverse event rates with HA
were found in the context of treatment for fecal incontinence or urinary incontinence.
b.
Over what time course does this local host response appear?
i. In the two aforementioned trials of adhesion barriers, adverse events occurred within 30 days of
surgery. In the two aforementioned trials of bulking agents, adverse events occurred within 12 months
of surgery.
2. Does the material elicit a persistent or exaggerated response that may lead to systemic signs or
symptoms – beyond known direct toxicity problems?
a.
What evidence exists to suggest or support this?
For 7 of 9 device categories, no systemic adverse events were statistically significantly more likely in
patients receiving HA device compared to patients received non-HA devices. The exceptions were adhesion
barrier and bulking agents.
b.
What are the likely systemic manifestations?
For adhesion barriers, creatinine was significantly higher in HA patients than in non-HA patients. For bulking
agents, fever was significantly higher in HA patients than in non-HA patients.
c. What is the observed timeline(s) for the systemic manifestations?

Material Performance Study - Hyaluronic Acid
|
15
Creatinine was significantly higher in HA patients on day 5 but not day 7 after surgery. Fever occurred
within the first 6 months after injection of the bulking agent.
d.
Have particular cellular/molecular mechanisms been identified for such manifestations?
The study authors suggested the HA barrier may cause a temporary increase in serum creatinine in the
early stage after surgery, but the effect is transitory. In the other study, authors did not speculate as to why
fever was more likely in HA-treated patients.
3. Are there any patient-related factors that may predict, increase, or decrease the likelihood and/or
severity of an exaggerated, sustained immunological/systemic response?
For adhesion barriers, one systematic review of laparoscopic gynecologic surgery
1
stated “Filmy mobile adhesions
resulted in the highest verbal pain scores followed by dense mobile adhesions, filmy fixed adhesions, and dense fixed
adhesions. Pain scores were highest when adhesions were between a segment of bowel and an ovary.
4. Are there any material-related factors that may predict, increase, or decrease the likelihood and/or
severity of an exaggerated, sustained immunological/systemic response?
For adhesion barriers, an RCT of laparoscopic colorectal and/or small bowel surgery stated, “The powder formulation
of HA/CMC used in this study is likely to be an important contributing factor for the increased frequency of adverse
events and serious adverse events.” “Due to the potential for greater diffusion of the powder formulation, the
authors speculate that migration away from the application site to anastomoses could have occurred in some cases.
Furthermore, over-hydration of the HA/CMC powder might have resulted in pooling of the resulting gel away from
the application site, raising the possibility of migration onto an anastomosis or provision of a nidus for abscess; such
migration to anastomoses might increase the rate of SSIs.”
5. What critical information gaps exist and what research is needed to better understand this issue?
All gaps listed here could benefit from future research.
a. For 7 of 9 device categories, no local adverse events were statistically significantly more likely in patients
receiving HA devices compared to patients receiving non-HA devices. This is predominantly because of a
lack of studies with strong design that investigated these events. The exceptions were adhesion barrier and
bulking agents.
b. For 7 of 9 device categories, no systemic adverse events were statistically significantly more likely in
patients receiving HA device compared to patients received non-HA devices. This is predominantly because
of a lack of studies with strong design that investigated these events. The exceptions were adhesion barrier
and bulking agents.
c. Only one study related to HA as a material was identified. However, because treated patients received HA
as well as chondroitin sulfate, one cannot determine whether the reported adverse events were due to HA
or chondroitin sulfate or other factors
d. For 8 of 9 device categories, only study involving adhesion barriers investigated the cellular or molecular
mechanisms for systemic manifestations.

Material Performance Study - Hyaluronic Acid
|
16
Project Overview
FDA engaged ECRI to perform a comprehensive literature search and systematic review to identify the current state of
knowledge with regard to medical device material biocompatibility. Specific materials or topics were selected by FDA based on
current priority. For 2021, the following 18 topics have been chosen.
1. Magnesium (Mg)
2. Complications associated with Polypropylene Mesh in Pre-, Peri-, and Post-Menopausal Women
3. Polytetrafluoroethylene (PTFE)
4. Acrylics 1: PMMA
5. Acrylics 2: pHEMA
6. Acrylics 3: Cyanoacrylates (PET)
7. Correlations between complications with polypropylene mesh and surgical procedure/anatomical location and
chemical/mechanical device properties
8. Dimethacrylates, Trimethacrylates (EDMA, EGDMA, TEGDMA, PEGDMA), and glycerol methacrylate (bis-GMA)
9. Polyethylene glycol (PEG)
10. Other Fluoropolymers (PFPE, PVDF, PVDF-HFP, PCTFE)
11. Silver
12. Small Molecule Per- and polyfluoroalkyl substances (SM-PFAS)
13. Hyaluronic Acid (HA) - Muscle/Skeletal Applications
14. Hyaluronic Acid (HA) - Dermal, Facial, and Eye Applications
15. Hyaluronic Acid (HA) – Adhesion Barriers
16. Polycaprolactone (PCL)
17. Zirconia
18. Nitinol
The systematic review was guided by key questions mutually agreed upon by FDA and ECRI. Data were extracted from
literature articles and ECRI surveillance databases accordingly.
Key Questions
1. What is the typical/expected local host response to Hyaluronic Acid?
a.
Can that response vary by location or type of tissue the device is implanted in or near?
b.
Over what time course does this local host response appear?
2. Does the material elicit a persistent or exaggerated response that may lead to systemic signs or symptoms – beyond
known direct toxicity problems?
a.
What evidence exists to suggest or support this?
b.
What are the likely systemic manifestations?
c.
What is the observed timeline(s) for the systemic manifestations?
d.
Have particular cellular/molecular mechanisms been identified for such manifestations?
3. Are there any patient-related factors that may predict, increase, or decrease the likelihood and/or severity of an
exaggerated, sustained immunological/systemic response?
4. Are there any material-related factors that may predict, increase, or decrease the likelihood and/or severity of an
exaggerated, sustained immunological/systemic response?
5. What critical information gaps exist and what research is needed to better understand this issue?

Material Performance Study - Hyaluronic Acid
|
17
If data did not exist to sufficiently address these questions, a gap was noted in this report. These gaps could represent areas
of further research.
Safety Profiles were written for the six materials listed above to include the summary of key findings from the systematic
review and surveillance search and are included in this report.
Literature Search and Systematic Review Framework
The ECRI-Penn Evidence-based Practice Center (EPC) conducts research reviews for the Agency for Healthcare Research and
Quality (AHRQ) Effective Health Care (EHC) Program. ECRI’s scientific staff within our Center for Clinical Excellence has
authored hundreds of systematic reviews and health technology assessments on 3,500+ technologies/interventions for ECRI’s
public- and private-sector clients. In addition to this work, ECRI staff have coauthored several methods papers on evidence
synthesis published on the AHRQ Effective Health Care website and in peer-reviewed journals.
For this project, the clinical and engineering literature was searched for evidence related to biocompatibility of each material.
Searches of PubMed/Medline and Embase were conducted using the Embase.com platform. Scopus was used initially to search
nonclinical literature; however, it was determined that the retrieved citations did not meet inclusion criteria and that database
was subsequently dropped from the search protocol. Search limits included publication dates between 2011 and 2021 and
English as the publication language. ECRI and FDA agreed on appropriate host and material response search concepts as
follows:
• Material Response
o Strength
o Embrittlement
o Degradation
o Migration
o Delamination
o Leaching
• Host Response
o Local
Inflammation
Sensitization
Irritation
Scarring/fibrosis
•
Keloid formation
•
Contracture
Ingrowth
Erosion
o Systemic
Cancer
Inflammation
Immune Response
Fatigue
Memory Loss
Rash
Joint Pain
Brain Fog
Search strategies were developed for each concept and combined using Boolean logic. Several search approaches were used
for comprehensiveness. Strategies were developed for devices of interest as indicated by FDA as well as the material-related
strategies. Each of these sets were combined with the material and host response strategies. Detailed search strategies and
contextual information are presented in Appendix B. Resulting literature was screened by title review, then abstract review,
and finally full article review. Data were extracted from the articles meeting our inclusion criteria to address the key questions
for each material.

Material Performance Study - Hyaluronic Acid
|
18
ECRI Surveillance Search Strategy
There are four key ECRI sources for medical device hazards and patient incidents. These databases were searched by key
terms and device models. Relevant data were extracted to address the key questions agreed upon by FDA and ECRI. Patient
demographics were extracted when available. All data presented were redacted and contain no protected health information
(PHI).
ECRI surveillance data comprise ECRI Patient Safety Organization (PSO) event reports, accident investigations, problem
reporting network (PRN) reports, and alerts. The PSO, investigations, and PRN reports included in this report include mostly
acute patient events. We rarely find chronic conditions or patient follow-up reports, which are more prevalent in the clinical
literature. Complications are reported directly by clinical staff; thus, reports vary greatly in the level of detail provided.
ECRI Patient Safety Organization (PSO)
ECRI is designated a Patient Safety Organization by the U.S. Department of Health and Human Services and has collected
more than 3.5 million serious patient safety events and near-miss reports from over 1,800 healthcare provider organizations
around the country. Approximately 4% of these reports pertain to medical devices. Most of these reports are acute (single
event) reports and do not include patient follow-up. These data were filtered by complication, and relevant reports were
included in the analysis. “Harm Score” refers to the National Coordinating Council Medication Error Reporting and Prevention
(NCC MERP) taxonomy of harm, ranging from A to I with increasing severity (see
Figure 1). The entire PSO database was
included in the search, with reports ranging from year 2004 through August 2021, unless otherwise noted.
Figure 1. NCC MERP “harm score,” which is now regularly used by patient safety organizations.
Category A (No Error)
Circumstances or events that have the capacity to cause error.
Category B (Error, no harm)
An error occurred, but the error did not reach the patient (an “error of omission” does reach the patient).
Category C (Error, no harm)
An error occurred that reached the patient but did not cause patient harm.
Category D (Error, no harm)
An error occurred that reached the patient and required monitoring to confirm that it resulted in no harm to the patient and/or
required intervention to preclude harm.
Category E (Error, harm)
An error occurred that may have contributed to or resulted in temporary harm to the patient and required intervention.
Category F (Error, harm)
An error occurred that may have contributed to or resulted in temporary harm to the patient and required initial or prolonged
hospitalization.
Category G (Error, harm)
An error occurred that may have contributed to or resulted in permanent patient harm.

Material Performance Study - Hyaluronic Acid
|
19
Category H (Error, harm)
An error occurred that required intervention necessary to sustain life.
Category I (Error, death)
An error occurred that may have contributed to or resulted in patient’s death.
Definitions
Harm: Impairment of the physical, emotional, or psychological function or structure of the body and/or pain resulting
therefrom.
Monitoring: To observe or record relevant physiological or psychological signs.
Intervention: may include change in therapy or active medical/ surgical treatment.
Intervention necessary to sustain life: includes cardiovascular and respiratory support (e.g., CPR, defibrillation, intubation).
Accident Investigation
ECRI has performed thousands of independent medical-device accident investigations over more than 50 years, including on-
site and in-laboratory investigations, technical consultation, device testing and failure analysis, accident simulation, sentinel
event and root-cause analyses, policy and procedure development, and expert consultation in the event of litigation. Our
investigation files were searched by keywords, and the search was limited to the past 10 years unless we found landmark
investigations that are particularly relevant to biocompatibility.
Problem Reporting Network (PRN)
For more than 50 years, ECRI’s Problem Reporting Network (PRN) has gathered information on postmarket problems and
hazards and has been offered as a free service for the healthcare community to submit reports of medical device problems or
concerns. Each investigation includes a search and analysis of the FDA MAUDE database for device-specific reports. Based on
our search findings, we may extend our analysis to all devices within that device’s FDA-assigned product code. The PRN
database was searched by keywords, and the search was limited to the past 10 years.
Healthcare Technology Alerts
We regularly analyze investigation and PRN data to identify trends in use or design problems. When we determine that a
device hazard may exist, we inform the manufacturers and encourage them to correct the problem. ECRI publishes the
resulting safety information about the problem and our recommendations to remediate the problem in a recall-tracking
management service for our members. The Alerts database contains recalls, ECRI exclusive hazard reports, and other safety
notices related to Medical Devices, Pharmaceuticals, Blood Products, and Food Products. This database was searched by
keywords and specific make and model, and the search was limited to the past 10 years.
Material Performance Study - Hyaluronic Acid
|
20
Safety Profile – Muscle/Skeletal Applications
Full Name: Hyaluronic Acid
CAS Registry Number: 9067-32-7
Safety Brief - Systematic Review Results
The systematic review included clinical and engineering literature on biocompatibility (i.e., host response and material
response) of Hyaluronic Acid used in medical devices. In addition to fundamental material biocompatibility, we focused on
specific devices known to be made of Hyaluronic Acid. The devices in Table 1 were recommended by FDA CDRH to guide ECRI
in searching this literature and ECRI’s surveillance data.
Table 1: Medical Devices Containing HA for Muscle/Skeletal Applications provided by FDA to Guide ECRI Searches
Regulatory Description
Product Code
Class
Acid, hyaluronic, intraarticular (viscosupplement)
MOZ
III
Filler, Bone Void, Calcium Compound
MQV
II
Filler, Bone Void, Osteoinduction (W/O Human Growth Factor)
MBP
II
Prosthesis, Toe, Hemi-, Phalangeal
KWD
II
The Safety Brief summarizes the findings of the literature search on toxicity/biocompatibility of Hyaluronic Acid.
Inclusion/exclusion criteria and quality of evidence criteria appear in Appendix A in the Appendices below. Quality of evidence
ratings reflected a combination of the quality of comparative data (study designs), quantity of evidence (number of relevant
studies), consistency of evidence, magnitude of effect, directness of evidence, and evidence for a dose response or response
over time. The search strategy appears in Appendix B1, and a flow diagram documenting inclusion/exclusion of studies
appears in Appendix C1. Summary evidence tables with individual study data appear in Appendix D1, and a reference list of
studies cited in the Safety Brief appears in Appendix E.
A summary of our primary findings is shown in Table 2. We then turn to a detailed discussion of research on Hyaluronic Acid
as a material as well as research on the various device categories.
In the discussion section, please note that a statement of “no difference” or “no significant difference” between
devices/materials does not imply equivalence between devices/materials, as studies with low numbers of patients or events
often lack sufficient statistical power to detect a difference between comparators. In addition, when we cite odds ratio(s), an
odds ratio >1 means that the rate was higher in the HA group than in the non-HA group.
Table 2: Summary of Primary Findings from Systematic Review – Muscle/Skeletal Applications
Application
Local Host
Responses/Device Events
Quality of Evidence
(local responses)
Systemic
Responses
Quality of Evidence
(systemic responses)
Hyaluronic Acid as a
material
No studies
Very low (no evidence)
No studies
Very low (no
evidence)
Material Performance Study - Hyaluronic Acid
|
21
Application
Local Host
Responses/Device Events
Quality of Evidence
(local responses)
Systemic
Responses
Quality of Evidence
(systemic responses)
Viscosupplementation –
knee
(26 human studies)
Arthralgia/joint pain,
Baker’s cyst, bleeding,
cutaneous vasculitis,
edema (injection-site,
peripheral), effusion,
erythema, heaviness of
injection, joint stiffness,
musculoskeletal pain, pain
at injection site, pain flare
ups, pain post-injection,
purulent aspirate, skin
reaction, swelling,
tenderness of knee joint
Moderate for swelling,
pain at injection site,
arthralgia/joint pain,
and effusion
Low for all other local
responses/device
events
Backache,
cardiac
disorders,
cellulitis,
death
possibly
device-
related, GI
disorders,
headache,
hypersensiti
vity
reaction,
musculoskel
etal and
connective
tissue
disorders,
nausea,
nervous
system
disorders,
paralysis,
rash, renal
and urinary
disorders,
respiratory/
thoracic/
mediastinal
disorders,
septic
shock,
septicemia,
skin
reaction,
skin and
subcutaneo
us tissue
disorders,
pseudosepti
c arthritis
(presenting
with
elevated
CRP, ESR,
PMN;
leukocytosis
and fever),
nasopharyn
gitis,
Low
Material Performance Study - Hyaluronic Acid
|
22
Application
Local Host
Responses/Device Events
Quality of Evidence
(local responses)
Systemic
Responses
Quality of Evidence
(systemic responses)
vascular
disorders,
vomiting
Viscosupplementation – hip
(4 human studies)
Hematoma, nausea, pain
flare ups, pain post-
injection, pruritus
Low
No studies
investigated
Very low
Viscosupplementation –
hand and ankle
(2 human studies)
Enlarged lymph node in
ipsilateral groin, pain at
injection site, pseudogout,
swelling
Low for swelling and
pain at injection site
Very low for all other
local responses/device
events
No studies
investigated
Very low
Viscosupplementation –
shoulder
(2 human studies)
Abscess, musculoskeletal
pain, pain at injection site
Low for
musculoskeletal pain
and pain at injection
site
Very low for all other
local responses/device
events
Cancer,
chest pain,
diarrhea, flu
symptoms,
headache
Very low
.
Viscosupplementation – TMJ
disorders
(2 human studies)
Discomfort post-injection,
ear pressure, pain at
injection site
Low for pain at
injection site
Very low for all other
local responses/device
events
Chills
Very low
Cartilage scaffold
(3 human studies)
Cyst formation, edema,
hematoma, persistent
pain, swelling
Low for swelling
Very low for all other
local responses/device
events
No studies
investigated
Very low
Bone putty/filler
No studies
Very low (no evidence)
No studies
Very low (no
evidence)
GI: gastrointestinal; TMJ: temporomandibular joint
Hyaluronic Acid as a Material
Our literature searches did not identify any studies of these devices that met inclusion criteria.
Viscosupplementation
Due to the primary focus on viscosupplementation in this evidence base, we provide results separately for five indications for
this device category (knee, hip, hand and ankle, shoulder, and TMJ disorders).
Viscosupplementation – knee
26 human studies (16 SRs
2-17
and 10 randomized controlled trials (RCTs)
18-27
). For further information see Table 1 Appendix D.
Material Performance Study - Hyaluronic Acid
|
23
Local Responses/Device Events (human studies)
IAHA:
2 SRs addressed the use of IAHA injections. The first SR
2
investigated fatal adverse events (AEs) from IAHA injections
listed in the MAUDE and Alternative Summary Reporting databases between 2014 and 2019. Brand names searched for in this
review included Durolane, Euflexxa, Gel-One/Gel One/GelOne, Gelsyn, Genvisc/Gen Visc, Hyalgan/Hyalgan LL, Hymovis,
Monovisc, Orthovisc, Sinovial, Supartz/Supartz FX, Suprahyal, Synojoynt, Synvisc/Synvisc-One, Triluron, Trivisc, and Visco-3.
Of 63 unique fatalities identified, 8 (12%) fatalities were possibly IAHA-related. Local responses affiliated with these cases
included pain after injection (3 patients), purulent aspirate (1 patient), and swelling of the knee (2 patients).
The second SR
3
investigated reporting of pseudoseptic arthritis, a rare complication after IAHA injection. 27 patients (28
knees) with acute pseudoseptic arthritis after HA injection (mostly Synvisc) were identified in 11 studies (5 single arm studies,
6 case reports). Results indicated that 22 cases (78.6%) of pseudoseptic arthritis presented within 24 hours of injection; 15 of
the 22 cases (68.2%) presented within the first 12 hours. Time from injection to presentation ranged from 1 hour to 9 days. 7
cases (25%) occurred after 2 injections, 5 cases (17.9%) after 3 injections, and 13 cases (46.4%) after ≥4 injections. All
patients presented with severe joint pain and effusion. 3 patients had synovial leucocyte elevation in the range typically
concerning for septic arthritis (above 50,000), and 16 patients had elevations in an inflammatory cell count range of 5,000 to
50,000. Results from a separate case report included progressive and increasing pain with obvious suprapatellar effusion at
12 hours post-injection; inflammatory symptoms resolving by 4 weeks.
IAHA versus saline/placebo:
8 studies (4 SRs
12-15
and 4 RCTs
20-23
investigated the safety of IAHA vs. saline/placebo in mostly
middle-aged women with mild-to-moderate OA. Overall, approximately 6,000 individuals enrolled in over 50 RCTs received
IAHA. The SRs and 1 RCT
23
investigated the safety of several IAHAs, while the remaining RCTs focused on one IAHA
(Synvisc,
20
Monovisc
21,22
). Dose was reported in 6 (75%) studies; and administrations ranged from 1 to 11 injections. Overall
mean followup was 6 months, and ranged up to 1 year
13,14
and 2 years.
12,23
Studies reported on overall rates for TRAEs,
13,14
AEs,
12,20
device-related AEs,
21
serious AEs (SAEs),
12,15,20,22,23
and non-serious
AEs.
15
• TRAEs: 2 SRs focused on TRAEs (not specified) up to 52 weeks.
13,14
The first SR
13
of 9 RCTs indicated significantly
increased odds of TRAEs with IAHA vs. saline (Odds ratio (OR) 1.78, 95% CI: 1.21 to 2.63), but no significant
differences in any severe AEs (OR 1.08, 95% CI: 0.50 to 2.31). The second SR
14
of 30 RCTs reported significantly
more TRAEs with IAHA in studies using ≥5 injections vs. saline (RR 1.70, 95% CI: 1.12 to 2.59), but no significant
difference for studies examining 1 injection or 2 to 4 injections vs. saline. This same SR reported no significant
difference in number of patients experiencing a TRAE (RR 1.13, 95% CI: 0.95 to 1.35).
• AEs: 2 studies reported similar overall AEs with IAHA vs. saline.
12,20
• Device-related AEs: 1 RCT reported device-related AEs occurred more frequently with Monovisc vs. saline (13 (7.1%)
vs. 10 (5.4%)).
21
• SAEs and non-serious AEs: 3 RCTs reported no SAEs
20,22,23
up to 104 weeks. 1 SR
11
reported no difference in risk of
SAEs (1.8% vs. 1.2%, RR 1.44, 95% CI: 0.91 to 2.26, p=0.12)
12,15
or non-serious AEs (415 HA, 375 placebo; RR
1.03, 95% CI: 0.93 to 1.15) up to 2 years.
Arthralgia and joint swelling were the most common AEs reported in 6 studies.
12,15,20-23
Arthralgia was reported in 5 (83%)
studies.
12,15,20-22
Rates were reported as similar versus saline and ranged from 1.3% to 3.8% up to 26 weeks.
20-22
2 RCTs
reported arthralgia with Synvisc and Monovisc was device-related.
20,22
Timing reported in 2 SRs (rates NR) was immediately
post-injection
15
and up to 3 days post-injection.
12
Joint swelling was reported in 4 (66%) studies;
12,15,20,21
one study indicated response was device-related.
20
Rates ranged from
1.1% to 3.7% with IAHA. 1 RCT reported a higher incidence of joint swelling with Synvisc (3.7% vs. 0.9%),
20
while another
RCT reported no significant difference in incidence of joint swelling (1.1% Monovisc, 0.5% saline).
21
Timing reported in 2 SRs
(rates NR) was immediately post-injection
15
and up to 3 days post-injection.
12
Less frequently reported AEs were injection site pain,
12,20,23
injection site swelling,
20,23
and joint stiffness.
21,23
Incidence of
injection site pain
was 0.5% with Synvisc,
20
and 21% with IAHA.
23
Timing was immediately after
23
and up to 3 days post
injection (rate NR).
12
Incidence of
injection site swelling
was 0.5% with Synvisc
20
and 21% with varying weights of IAHA.
23
Incidence of
joint stiffness
in the index knee ranged from 0.5% (with Monovisc)
21
to 7% (with varying weights of IAHA).
23
Rates were lower vs. saline (1.1%)
21
and timing was immediately post-injection.
23
Additional AEs reported by 1 study each included the following:

Material Performance Study - Hyaluronic Acid
|
24
• joint pain immediately post-injection
15
• injection site joint pain (0.5% with Synvisc, 0% placebo)
20
• device-related non-serious cutaneous vasculitis during 1st week post-injection
15
• peripheral edema with Synvisc (0.5% HA, 0% placebo)
20
• effusion immediately post-injection
15
)
• joint effusion up to 3 days post-injection
12
• injection site edema with Synvisc(0.5% HA, 0% placebo)
20
• injection site erythema (12% overall rate for all IAHA weights)
23
• joint tenderness immediately post-injection
15
• transient increase in pain immediately post-injection
15
IAHA versus platelet-rich plasma (PRP):
3 studies (1 SR
4
and 2 RCTs
18,19
) addressed this topic in 1,137 individuals with mild-
to-moderate knee osteoarthritis (OA). A majority of patients were females with mean age of 60 years. The sample size was
greater than 100 patients for the RCTs,
18,19
and ranged from 55 to 183 in the SR (mean 128 patients).
4
Dose was not
reported. Administrations were reported in the 2 RCTs as single injections of HA,
18
and 3 weekly injections of Hyalgan
(FidiaFarmaceutici S.p.A.).
19
Followup was 6 months
18
and 12 months.
4,19
Local responses reported in the SR
4
included pain and swelling immediately after injection. Of the 7 included RCTs (n= 908), 3
RCTs reported no significant differences in AEs, 2 studies reported significantly more post-injection pain and/or swelling with
PRP, while 2 studies reported no AEs.
The first individual RCT
18
reported no significant differences for mild-to-moderate AEs (musculoskeletal pain, swelling and
tenderness of the knee joint) at 6 months in 110 individuals (55 each arm) receiving single injections of HA or PRP.
Musculoskeletal pain occurred in 4 patients (3 (5%) HA, 1 (1.8%) PRP; timing NR). Mild-to-moderate swelling of the knee
joint occurred similarly at 6 weeks (100%), and a lower incidence with HA at 3 months (85% vs. 96%), and 6 months (87%
vs. 91%). Mild tenderness was reported in 3 patients (2 with HA at baseline and 6 weeks, 1 with PRP at baseline).
The second individual RCT
19
reported no significant differences in minor complications (stiffness and heaviness of injection
site) immediately post-injection in 13 of 119 patients (3 (6%) HA, 10 (20%) PRP-derived growth factor (PRGF)).
IAHA versus intra-articular oxygen-ozone:
One SR
5
of 4 RCTs addressed this topic in 298 individuals with end-staged knee OA.
Sample size ranged from 42 to 141 patients. The population was 75% female aged 60 to 71 years. Dose was reported as 10
mg/ml x 2.0 ml (3 studies), and 10 mg/ml x 2.5 ml (1 study). Frequency of injection was not reported. Local responses
included bleeding and skin reaction at 6 to 12 months. No significant differences were reported in incidence rate of AEs (risk
difference 0.006, 95% CI: -0.047 to 0.058).
IAHA versus IAHA (different weights):
2 SRs
6,7
including 40 RCTs compared the safety of IAHA of varying molecular weights in
individuals with knee OA. 1 SR reported the average molecular weight ranged from 500 to 3600 kDa with IAHA mostly being
administered in 3 injections.
7
Mean followup was 6 months in 1 SR reporting.
7
One SR
6
of 30 RCTs examined IAHA injections of high molecular weight (HMW), medium MW (MMW), and low MW (LMW),
and whether the products were processed through extraction of avian-derived molecules (AD-HA) or through bacterial
processes of biological fermentation (Bio-HA). 11 RCTs (n=2094) addressed HMW, 4 RCTs (n=621) addressed MMW, and 15
RCTs (n=2639) addressed LMW. Bio-HA and AD-HA treatments were administered to 1,776 and 3,070 patients, respectively.
Local responses from products such as Euflexxa, Synvisc, Supartz, Orthovisc, and Durolane included effusion after injection
and flare ups. Followup was NR.
Results based on weight: HMW products had the highest rate of injection site flare-ups. Significantly more injection
flare ups with HMW vs. MMW (13.73% vs. 3.31%) and HMW vs. LMW (13.73% vs. 10.73%). Significantly more flare
ups with LMW vs. MMW (10.73% vs. 3.31%). No significant difference was reported in effusion after injection based
on molecular weights (1.9% HMW, 1.7% MMW, 1.8% LMW).
Results based on process: Use of AD-HA products resulted in a significantly lower incidence of acute flare ups (3.0%
vs. 13.2%), but a significantly higher incidence of effusion (3.4% vs. 0.5%) versus Bio-HA.
Another SR
7
included 10 RCTs in a safety analysis comparing Synvisc of a relatively HMW versus IAHA with LMW (Artzal,
Orthovisc, Bio-HA, Hyalgan, Sinovial, Variofil, Hydros) in 2,616 patients. Authors reported no significant difference in TRAEs at
mean 26.8 weeks (14.7% Synvisc vs. 11.6% LMWHA; risk difference 0.02, 95% CI: -0.01 to 0.05; p=0.13) or number of
TRAEs (23.2% Synvisc vs. 16.3% LMWHA; risk difference 0.03, 95% CI: -0.01 to 0.07; p=0.14).

Material Performance Study - Hyaluronic Acid
|
25
IAHA versus anti-inflammatories
: Of 4 SRs addressing this comparison,
8-11
3 SRs examined local responses with IAHA,
8,10,11
while 1 SR only examined systemic responses (see below).
9
2 SRs examined IAHA versus NSAIDs.
8,11
The first SR of 6 RCTs
8
included 831 middle-aged patients with mild-to-moderate
knee OA. Sample size was 60 to 327 patients. Administrations were one injection (Durolane), 3 weekly injections (Synvisc, and
Suplasyn), and 5 weekly injections (Hyalgan and Suvenyl). Responses from 5 weeks to 26 weeks included arthralgia, injection
site pain, and lower leg effusion (1 (0.3%) HA, 2 (0.5%) NSAID). Results from a meta-analysis of 5 RCTs indicated:
• no significant difference in risk of serious AEs (1.2% vs. 0.9%, risk ratio 1.37, 95% CI: 0.26 to 7.14),
• a significantly lower risk of AEs with HA (19.8% vs. 29%; risk ratio 0.74, 95% CI: 0.59 to 0.94),
• a significantly higher risk of arthralgia with HA (8.1% vs. 2.9%).
The second SR of 5 RCTs (n=712) reported no IAHA-related AEs up to 12 weeks.
11
TRAEs were reported in one SR of 12 RCTs (n=1794)
10
comparing IAHA with intraarticular corticosteroids (CS; triamcinolone
hexacetonide, methylprednisolone acetate, 6-methylprednisolone acetate, triamcinolone acetonide). Sample size was 41 to
391 patients. IAHA doses ranged from 16 to 60 mg, while administrations ranged from 1 to 5 weekly injections. Knee pain,
joint swelling, and joint stiffness were reported from 1 to 6 months. Results indicated significantly more TRAEs with HA (746
events vs. 606 events; risk ratio 1.66, 95% CI: 1.34 to 2.06) which may have been caused by the higher injection frequency
IAHA versus miscellaneous treatments:
6 studies (2 SRs,
16,17
and 4 RCTs
24-27
addressed this comparison. One study each
compared IAHA with usual care,
26
debridement,
27
cooled radiofrequency ablation (CRFA),
24
acupotomy,
16
and
polydeoxyribonucleotide (PDRN).
17
One study compared IAHA with physical therapy (PT), dextrose prolotherapy, and
botulinum neurotoxin.
25
Over 100 patients were examined in each study; individuals with mild-to-moderate knee OA,
26,27
or at
least moderate knee OA
24,25
in studies reporting. Dose was reported in all studies, and administrations ranged from 1 to 5
injections. Followup was mostly 6 months and 12 months.
One study noted no device-related responses with Hyalgan vs. PT, dextrose prolotherapy, and botulinum neurotoxin.
25
2 RCTs
reported fewer TRAEs with IAHA. One RCT
24
noted a TRAE rate of 14% with Synvisc-One versus 19% TRAE rate with CRFA up
to 6 months. While procedural pain (3 (3%) in both arms), and post-procedural pain (3 (3%) with Synvisc-One, 7 (8%) with
CRFA) occurred in both arms, Baker’s cyst (a fluid-filled cyst that causes a bulging and feeling of stiffness behind the knee)
only occurred in 2 (2%) patients with Synvisc-One. The other RCT (n=120)
27
reported pain at injection site in 8 (13.4%)
patients after IAHA vs. pain and mild effusion in 13 (26%) patients after debridement up to 6 months.
The remaining 3 studies reported a similar incidence of TRAE/AEs. One SR of 5 RCTs
17
reported a similarly low incidence of
TRAEs (unspecified) with HA and PDRN up to 12 months. One SR of 4 RCTs (n=309) reported AE incidence rates of 2.2% with
HA, and 4.3% with acupotomy up to 6 months. AEs included redness and swelling post-injection in 3 patients with each
treatment.
16
1 RCT comparing 3 weekly injections of Synvisc (HMW-HA) vs. usual care
26
in 156 patients reported similar
overall TRAEs (77 Synvisc, 79 UC). Events, described as flare-ups or “other”, were reported at 6 weeks, 13 weeks, 26 weeks,
39 weeks, and 52 weeks. Flare-up rates with Synvisc were highest at 6 weeks (36%) and lowest at 52 weeks (6%).
Systemic Responses
IAHA:
Both SRs addressing this topic reported systemic responses. The first SR
2
identified 63 unique fatalities, 8 fatalities were
possibly IAHA-related. Of the 8 fatalities, 4 patient’s experienced local responses such as pain and swelling (see above) prior
to death. 1 death involved a suicide due to worsening pain. The remaining 4 patients experienced the following systemic
responses prior to death:
• septic shock, followed by paralysis, and death 4 months post-injection.
• necrotizing fasciitis after starting chemotherapy and receiving an HA injection.
• septicemia, cause of death unknown.
• cellulitis.
Authors noted that the available information was “insufficient to make a firm determination of causality.”
The second SR
3
reported results from 27 patients (11 studies) with acute pseudoseptic arthritis after mostly Synvisc
injections. Systemic responses included elevated CRP above 50, elevated ESR, elevated PMNs above 75% in 10 (37%)
patients, fever in 6 (22%) patients, and leukocytosis above 10,000 in 4 (15%) cases. Results from a separate case report
included a cell count of 123,260 and white blood cell count of 15.5.
Material Performance Study - Hyaluronic Acid
|
26
IAHA versus saline/placebo:
Of the 8 studies addressing this comparison, 3 studies investigated systemic responses.
13,15,22
1
SR, focused on TRAEs,
13
reported no significant differences in any system-organ class(SOC)-related AEs (GI, cardiac, vascular,
respiratory, nervous system, skin and subcutaneous tissue disorders, musculoskeletal, renal and urinary disorders, and
hypersensitivity reaction).
• GI disorders: 69 HA, 83 placebo; OR 0.81, 95% CI: 0.52 to 1.27
• cardiac disorders: 5 HA, 4 placebo; OR 1.25, 95% CI: 0.36 to 4.41
• vascular disorders: 5 HA, 3 placebo; OR 1.70, 95% CI: 0.39 to 7.29
• respiratory, thoracic and mediastinal disorders: 71 HA, 52 placebo; OR 1.21, 95% CI: 0.82 to 1.78
• nervous system disorders: 66 HA, 55 placebo; OR 1.15, 95% CI: 0.77 to 1.70
• skin and subcutaneous tissue disorders: 22 HA, 10 placebo; OR 1.71, 95% CI: 0.52 to 5.63
• musculoskeletal and connective tissue disorders: 145 HA, 149 placebo; OR 0.99, 95% CI: 0.71 to 1.39
• renal and urinary disorders: 6 HA, 12 placebo; OR 0.54, 95% CI: 0.21 to 1.41
• hypersensitivity reaction: 23 HA, 19 placebo; OR 0.64, 95% CI: 0.05 to 7.94
IAHA versus PRP:
One RCT reported nasopharyngitis in both arms (2 HA, 1 PRP); and backache (2 HA) and headache (1) only
with HA.
18
One SR investigated but did not identify any systemic responses.
4
IAHA versus anti-inflammatories
: 3 of 4 SRs addressing this comparison investigated systemic responses. One SR
9
comparing
IAHA with intraarticular methylprednisolone only reported on systemic responses. Results from 4 of 5 included RCTs indicated
headache, nausea, and vomiting with no significant difference in AE incidence up to 52 weeks (risk difference -0.042, 95% CI:
-0.092 to 0.009). One SR
8
(n=831) reported a significantly higher risk of headache (8.4% vs. 4.4%) with HA and a rare
occurrence of cardiac-related complications (1 (0.3%) HA, 0 NSAID). Lastly, one SR (n=1794) did not identify any treatment-
related systemic responses.
10
1 RCT reported rash in 1 patient with Monovisc, and indicated this response was device-related.
22
Lastly, 1 SR of 10 RCTs
indicated 1 serious AE attributed to HA was skin reaction characterized by peeling of skin on hands and toes in 1 patient. This
reaction occurred 8 days post-injection.
15
IAHA versus miscellaneous treatments:
1 of 6 studies addressing this comparison reported systemic TRAEs included GI
complaints from 6 weeks to 52 weeks. Rates ranged from 3% (at 26 weeks) to 7% (at 6 weeks).
26
Overall Quality of Evidence
The evidence for swelling, pain at injection site, arthralgia/joint pain, and effusion was consistently reported across high
quality studies, in agreement with reporting with other HA categories (e.g., viscosupplementation-hand and ankle,
viscosupplementation-shoulder, viscosupplementation-TMJ; and cartilage scaffold), so the quality of evidence is moderate. For
other local responses/events and systemic responses, the quality of evidence is low due to the large evidence base of high
quality studies.
Viscosupplementation - hip
4 human studies (4 SRs
28-31
). For further information see Table 2 Appendix D.
Local Host Responses (human studies)
One SR
28
included 5 RCTs (n = 591) comparing HA (n=298) with placebo injection (n=293) for hip OA. The HA products used
in the studies were Durolane 3 ml, hylan G-F 20 6 ml, Hyalubrix 8 ml, Hyalgan 6 ml, and Adant 2.5 ml, and follow-up ranged
from 56 to 182 days. No meta-analysis of AEs was performed. The SR lists numbers of patients in each study who
experienced slight or moderate pain flare during or after injection, permitting calculation of the following pooled AE rates: 21
of 298 patients in the HA groups (7%) and 7 of 293 patients in the placebo groups (2.4%). Pruritus and hematoma at the
injection site was reported in 1 patient each in the HA group.
Another SR
29
included 9 RCTs (n=1,164) comparing HA (n=558), methylprednisolone (n=201), PRP (n=115), mepivacaine
(n=20), and placebo (n=270) for hip OA. The HA products used in the studies were Durolane 3 ml, Hyalubrix 5 ml (2 studies),
Synvisc 6ml, Hyalubrix 8 ml, Hyalgan 6 ml, Adant 2.5 ml, and hylan G-F 20 2 ml; follow-up ranged from 2 to 12 months. Meta-
analysis of overall AEs was performed for comparisons of HA with placebo and methylprednisolone; otherwise, rates of overall
AEs were reported by study, permitting calculation of pooled rates. A 29.0% overall AE rate was reported for patients in the
HA groups across 9 studies (range: 0% to 49.7%). The rate was 23.7% for patients in the placebo groups across 4 studies

Material Performance Study - Hyaluronic Acid
|
27
(range: 0% to 34.9%), 8.7% for patients in the PRP groups across 3 studies (range: 0% to 20%), 38.3% for patients in the
methylprednisolone groups across 3 studies (range: 0% to 48.7%), and 5% for patients in the mepivacaine group in 1 study.
In the meta-analyses, no statistically significant differences in rates of overall AEs were found for HA versus placebo or for HA
versus methylprednisolone.
Another SR
30
included 4 RCTs (n=303) comparing HA (n=148) with PRP (n=155) for hip OA. The SR did not specify the
products used in the studies. Follow-up time was 12 months. Meta-analysis revealed a relative risk of nausea of 1.15 (95% CI:
0.34 to 3.93) for PRP relative to HA. One included study reported significantly higher post-injective pain for PRP than for HA.
Another SR
31
included 6 RCTs (n=630) comparing HA (n=291) with placebo (n=114) or methylprednisolone (n=207) for hip
OA. The HA products used in the studies were hylan G-F 20 2 ml, Hyalubrix 8 ml, Ostenil 2 ml, Durolane 3 ml, Adant 2.5 ml,
and Hyalgan 6 ml. (One included study compared Ostenil with hylan G-F 20; the SR appears to have treated hylan G-F 20 as a
control treatment in this study, although it treated the same product as HA in another included study.) Follow-up ranged from
56 to 180 days. Four of the six included studies reported AEs by treatment group; meta-analysis compared overall AEs in the
HA groups versus the control groups (apparently both placebo and control treatments) and obtained a relative risk of 0.94
(95% CI 0.41 to 2.20).
Systemic Responses
We did not identify any studies reporting systemic responses to HA as viscosupplementation in hips.
Overall Quality of Evidence
The 4 systematic reviews included high quality studies with a high number of patients, and most outcomes were in agreement
with other device categories. Since reporting of specific responses was limited to one systematic review each, the overall
quality of evidence was rated low. Since systemic responses were not investigated in these studies, the evidence is very low.
Viscosupplementation – hand and ankle
2 human studies (2 SRs
32,33
). For further information see Table 3 Appendix D.
Local Responses/Device Events (human studies)
One SR
32
included 24 studies (n = 844) of intra-articular injective treatments for ankle lesions, 18 of which examined HA. Of
the 24 studies, 8 were RCTs, 1 was a nonrandomized comparative study, and 15 were single-arm studies. The HA products
used in the studies were Euflexxa 6 ml (2 studies), Durolane 1ml, Suplasyn 6 ml, Hanox M-XL 2.2 ml, Hyalgan 2 ml to 10 ml
(4 studies), Synvisc 2 ml to 6 ml (3 studies), Supartz 2.5 ml to 12.5 ml (2 studies), Orthovisc 1 ml to 3 ml, Adant 7.5 mg, and
unspecified HA products 6 ml to 12.5 ml (2 studies). 581 patients received HA; other treatments were PRP (n = 83),
methylprednisolone (n = 48), botulinum toxin type A (n = 38), prolotherapy (n = 27), exercise therapy (n = 15),
mesenchymal stem cells (n = 6), and placebo/saline (n = 46). Follow-up ranged from 1 to 45.5 months. AEs with HA in 1
patient each included enlarged lymph node in ipsilateral groin, osteochondritis dissecans (not considered treatment-related),
and pseudogout. The SR also noted that “in some cases, pain and swelling were reported at the injection site soon after the
injection.”
The other SR
33
included 13 studies (n = 809) of intra-articular therapies for hand OA; 9 studies examined HA. Of the 13
studies, 12 were RCTs and one was a nonrandomized comparative study. The HA products used in the studies included hylan
G-F 20 1 ml to 2 ml (3 studies) and unspecified sodium hyaluronate products 1.5 ml to 3 ml (6 studies). 360 patients received
HA; other treatments were corticosteroids (n = 280), dextrose (n = 30), infliximab (n = 10), and placebo (n = 172). (These
numbers total more than 809 because two studies were within-subject and each patient in those studies [33 HA/placebo, 10
infliximab/placebo] is counted as having received both treatments.) Follow-up time was 24 weeks. Of the nine studies
including HA, one did not report numbers of patients experiencing AEs. In the 8 remaining studies, AE rates were as follows:
HA, 11.5%; corticosteroid, 9.0%; placebo, 2.7%. Numbers of patients with specific types of AE were not reported, either
overall or by group; AEs were described as minor, and included surgeries unrelated to study medication, pain and local
swelling, skin and nail abnormalities, heat and/or redness, and unspecified “local AEs.”
Systemic Responses
We did not identify any studies reporting systemic responses to HA as viscosupplementation in hands and ankles.
Material Performance Study - Hyaluronic Acid
|
28
Overall Quality of Evidence
The evidence for swelling and pain at injection site was reported in 2 SRs that included numerous high-quality studies. These
outcomes were also in agreement with other indications for viscosupplementation (e.g., knee, shoulder, TMJ), so we rated the
quality of evidence as low. For other local responses and systemic responses, the quality of evidence is very low.
Viscosupplementation – shoulder
2 human studies (2 SRs
34,35
). For further information see Table 4 Appendix D.
Local Host Responses (human studies)
One SR evaluated the safety of HA in patients 18 years of age and older with glenohumeral OA. Follow-up time ranged from
12 weeks to 3 years and sample size for the HA arms of the studies varied from 15 to 265, with 50.5% to 76% male patients.
Common AEs from 13 of the 15 included studies (5 RCTs, 10 single arm) were musculoskeletal pain, and pain at injection with
HA.
34
Serious AEs such as severe musculoskeletal pain and abscess were also reported. Similar AEs were reported in
individuals receiving intra-articular injections of corticosteroids or saline. The overall pooled AE rate (local and systemic) was
33.92% (406 of 1197 patients) and the serious AE rate was 5.35% (64 of 1197). Most events were deemed by study
investigators to not be product-related.
Another SR of 4 RCTs investigated the safety of HA administration versus conventional adhesive capsulitis (AC) therapies
(e.g., intra-articular corticosteroid injection and physical therapy) versus HA administration plus conventional therapy
regimens in patients with AC.
35
Follow-up ranged from 2 weeks to 6 months. Doses ranged from 20 to 30 mg, while
administrations ranged from 2 to 8 total injections. Mean patient age ranged from 54.5 to 64.2 years, and 69% were female.
2 studies reported no major AEs, and 1 study reported intra-procedural pain in 12 out of 45 participants undergoing capsular
distension combined with intra-articular HA injection. It was unclear if the pain was a result of the capsular distension
procedure, the intra-articular HA injection, or both.
Systemic Responses
Diarrhea, flu symptoms, and headache were commonly reported (data not provided) in 1 SR with up to 3 years followup.
34
Serious AEs such as chest pain and cancer were also reported (N not reported). Most responses were deemed by study
investigators to not be product related.
Overall Quality of Evidence
Musculoskeletal pain and pain at injection site were both commonly reported in 2 high-quality studies and in agreement with
other indications for viscosupplementation (e.g., knee, hand and ankle, TMJ), so we rated the quality of evidence as low. For
other local responses and systemic responses, the quality of evidence is very low.
Viscosupplementation – TMJ disorders
2 human studies (2 SRs
36,37
). For further information see Table 5 Appendix D.
Local Responses/Device Events (human studies)
Studies analyzed HA injections as viscosupplementation versus other types of intra-articular injections (corticosteroid [CS],
PRP, or platelet-rich growth factor [PRGF]) in the treatment of TMJ disorders. Dose ranged from 0.5 to 5 ml with single
administration in both studies. Follow-up was 1 week to 8 years
36
and 12 months.
37
Sample size was 13 to 102 patients with a
mean age of 25 to 50 years.
Pain at injection site: Three RCTs included in a SR
36
reported TMJ pain post-injection with HA compared to CS. Zero percent to
37% of patients receiving HA reported pain post-injection versus 0 to 70% patients reporting pain after CS injection (OR 2.98,
95% CI: 0.08 to 111.17). Overall, the review found no relevant difference between the HA and CS treatment groups in terms
of adverse events. The SR comparing HA to PRP or PRGF
37
included one RCT reporting AEs, assessing that 60% percent of
patients receiving HA reported pain during injection versus 88% of patients receiving PRP or PRGF.
Ear pressure: One RCT included in a SR
36
reported 5% of HA patients with ear pressure compared to 10% of CS patients (OR
= 2.11, 95% CI: 0.18 to 25.35) at two-week follow-up.

Material Performance Study - Hyaluronic Acid
|
29
Post-operative discomfort: The SR comparing HA to PRP or PRGF
37
included one RCT reporting AEs, assessing that 8% of
patients receiving HA reported post-operative discomfort versus 76% of patients receiving PRP or PRGF.
Systemic Responses
An RCT included in a SR
36
reported slight chills at 1 week follow-up for HA compared to CS. Zero percent of CS patients
reported chills versus 5.7% of patients receiving HA (OR=0.24, 95% CI: 0.01 to 5.90; p=0.36). Overall, the review found no
relevant difference between the HA and CS treatment groups in terms of AEs.
Overall Quality of Evidence
Pain at injection site was reported by both SRs and in agreement with other indications for viscosupplementation (e.g., knee,
shoulder, hand and ankle), so we rated the quality of evidence as low. For other local responses and systemic responses, the
quality of evidence is very low.
Cartilage scaffold
3 human studies (1 RCT,
38
and 2 nonrandomized comparative studies
39,40
). For further information see Table 6 Appendix D.
Local Host Responses (human studies)
The RCT compared polyglycolic acid and hyaluronan matrix-augmented bone marrow stimulation (m-BMS) with
Chondrotissue (BioTissue AG) after microfracture (MF) to MF alone in 24 patients with grade III/IV International Cartilage
Repair Society cartilage degradation.
38
One patient in the m-BMS group reported an infected hematoma up to 108 weeks.
Three patients in the m-BMS group reported mild swelling after 2 weeks, which resolved by 6 weeks; swelling did not occur in
the MF group. There was 1 severe effusion after 6 weeks in the MF group, and none in the m-BMS group. Neither group had
allergic reactions (moderate or severe).There were no events that were more likely in the group receiving HA (the m-BMS
group).
Two nonrandomized comparative studies compared HA-based cell-free scaffold with Hyalofast (Anika Therapeutics) with a
chitosan-glycerol phosphate/blood implant (BST-CarGel®, Piramal Life Sciences)
39
or MF alone
40
in patients with focal
osteochondral lesions of the knee joint (Outerbridge grade III or IV).
The first study enrolled 21 patients in the Hyalofast scaffold group and 25 in the BST-CarGel implant group.
39
At mean 24
months follow-up, edema or cyst formation in the subchondral bone was detected in 8 (38%) knees with Hyalofast, and 10
(40%) knees with BST-CarGel). One patient from each group had persistent pain as well as early degenerative changes of the
knee joint and planned to undergo replacement surgery at the latest follow-up. Deep venous thrombosis, septic arthritis,
neurovascular complication, or intra-articular adhesion were not detected in any patients.
The second study enrolled 24 patients in the MF group (mean age 43±6.8 years) and 19 in the Hyalofast group (mean age
40±9.8 years), 62.8% female overall.
40
1 patient from the HA scaffold group and 2 patients from the MF group had persistent
pain as well as early degenerative changes of the knee joint up to mean 25 months.
Systemic Responses
We did not identify any studies investigating systemic responses to HA as cartilage scaffolds.
Overall Quality of Evidence
None of the three studies found any higher rates in groups receiving HA, but they may not have been large enough to detect
effects. The evidence for swelling was reported in 1 high-quality study and in agreement with reporting with other HA
categories (e.g., viscosupplementation-knee, and viscosupplementation-hand and ankle), so the quality of evidence is low. For
other local responses/device events and systemic responses (no studies investigated), the quality of evidence is very low.
Bone putty/filler
Our literature searches did not identify any studies of these devices that met inclusion criteria.
ECRI Surveillance Data
Refer to Appendix F for a list of devices that guided our searches of ECRI Surveillance Data.
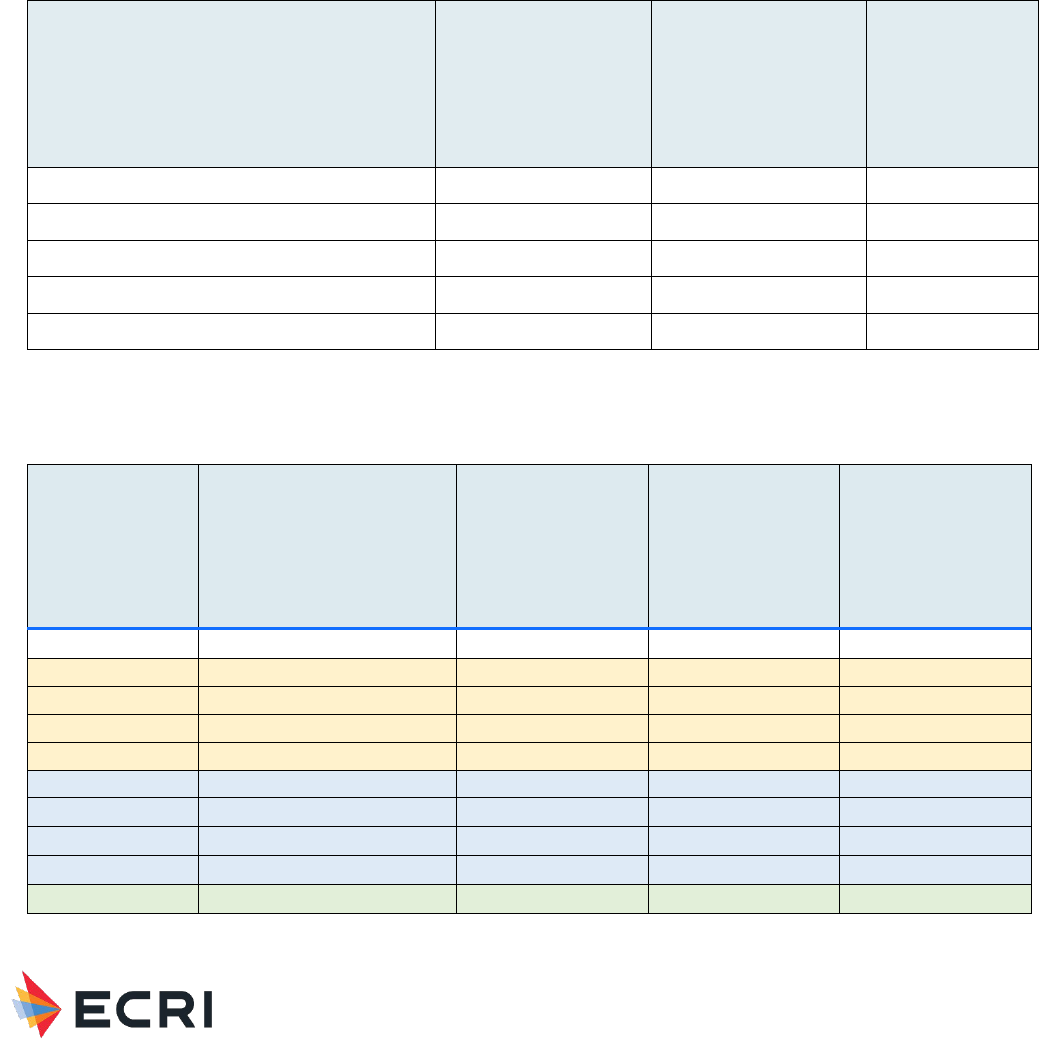
Material Performance Study - Hyaluronic Acid
|
30
Patient Safety Organization
Search Results: ECRI PSO identified 115 reports that involved HA related to muscle/skeletal applications that occurred
between April 2016 and November 2021. Seven of these involved pertinent events. The events included: 1) Wrong side
procedure - 2 (28.5%), 2) Wrong product - 2 (28.5%), 3) Product expired - 2 (28.5%), 4) Wrong time – 1 (14.2%). Table 3
and Table 4 outline the complications and harm scores of these events, respectively.
All individual PSO event reports are redacted and included in Appendix F.
Table 3" Complications in HA-related PSO Event Reports – Muscle/Skeletal Applications
Complication
Viscosupplement
Bone Filler
Total
Wrong side procedure
2
2
Wrong product
2
2
Product expired
1
1
2
Wrong time
1
1
Total
6
1
7
Table 4: Harm Score Associated with HA-related PSO Event Reports – Muscle/Skeletal Applications
Harm Scores
(NCC-MERP)
Harm Scores (NCC-MERP)
Viscosupplement
Bone Filler
Total
A
No Error
B1
Error, No Harm
B2
Error, No Harm
C
Error, No Harm
4
1
5
D
Error, No Harm
1
1
E
Error, Harm
1
1
F
Error, Harm
G
Error, Harm
H
Error, Harm
I
Error, Death
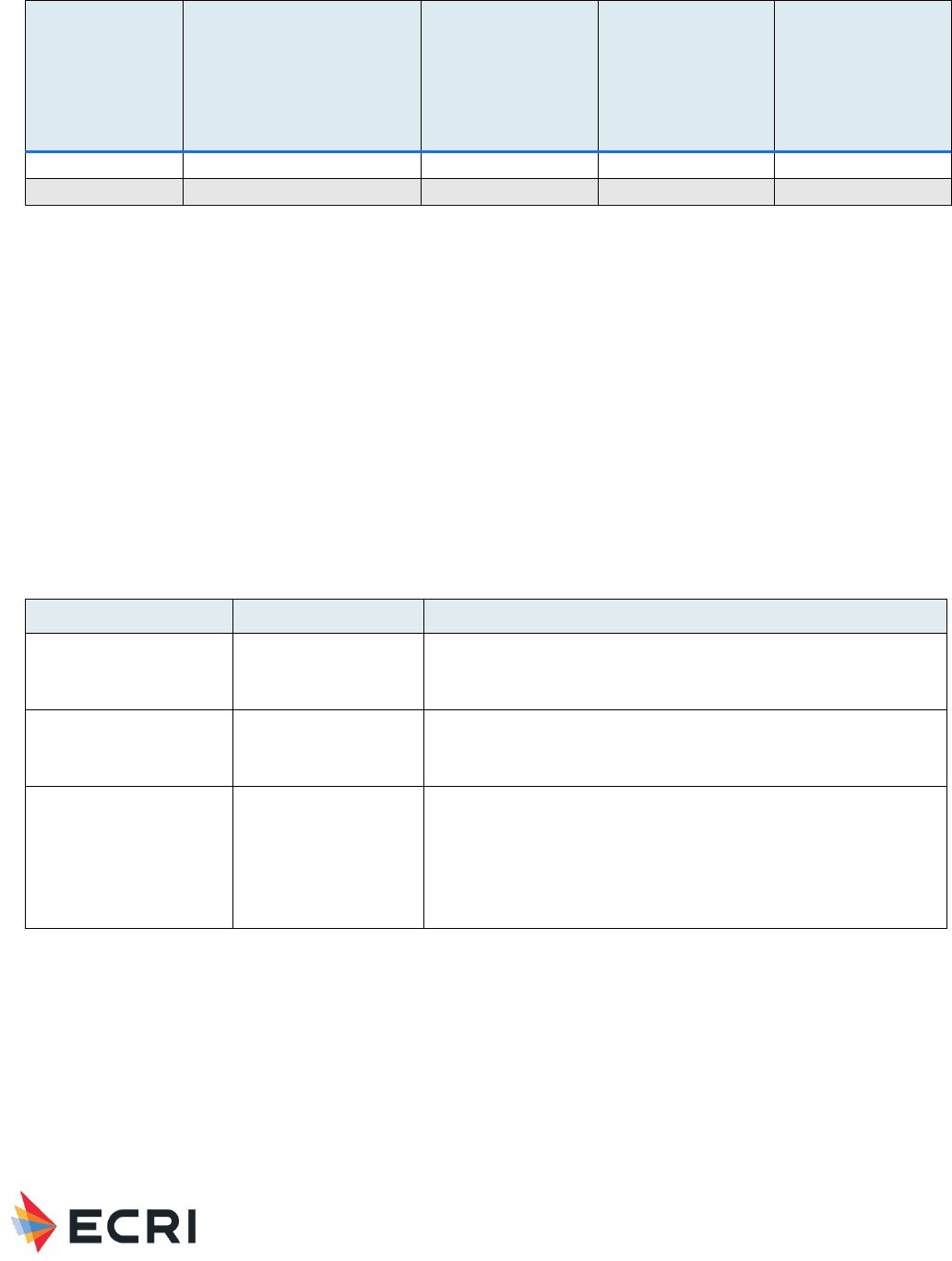
Material Performance Study - Hyaluronic Acid
|
31
Harm Scores
(NCC-MERP)
Harm Scores (NCC-MERP)
Viscosupplement
Bone Filler
Total
NULL*
Total
6
1
7
*Harm score was not reported
Accident Investigations
Search Results: Zero investigations were recovered from the accident investigations database.
ECRI Problem Reports
Search Results: The search returned zero reports submitted by ECRI members.
Healthcare Technology Alerts
Search Results: The search returned 5 manufacturer issued alerts describing problems with adverse events, contamination,
packaging issues, and impartially filled products, summarized in Table 5.
Table 5: Summary of Regulatory and Manufacturer Alerts - Muscle/Skeletal Applications
Device Type
# Alerts
Reported Problem
Viscosupplemenation:
MOZ (Acid, Hyaluronic,
Intraarticular)
2 manufacturer issued
• Reports of post-injection pain and swelling
• Contaminated product may lead to infection
Bone Filler:
MQV (Filler, Bone Void,
Calcium Compound)
2 manufacturer issued
• Impartially filled tubes may delay surgery
• Unsealed Tyvek packaging
Bone Filler:
MQV (Filler, Bone Void,
Calcium Compound);
MBP (Filler, Bone Void,
Osteoinduction (w/o
Human Growth Factor)
1 manufacturer issued
• Incomplete outer packaging seal
Potential Gaps
ECRI surveillance searches reflect mostly acute patient incidents that involved medical devices made of Hyaluronic Acid. Areas
of particular concern involve incidents that result in direct tissue exposure to the material if there is moderate to high-quality
evidence of acute or systemic reaction to this exposure, as determined by the systematic review. Topics with very low or low
quality of evidence represent areas of potential gaps in the literature. If the literature revealed areas of new concern (e.g.,
systemic response to long-duration contact) and there is little supporting evidence, these are considered gaps.

Material Performance Study - Hyaluronic Acid
|
32
HA as a Material
There were no studies that met inclusion criteria for HA as a material indicating an area of future research.
Viscosupplementation
14 HA products were investigated in 36 studies addressing five various indications (knee, hip, hand/ankle, shoulder, and TMJ
disorders). Hyalgan and Synvisc were investigated in 15 studies and 16 studies, respectively. 9 products (Monovisc, HYADD,
Sinovial, Gel-One, Supartz, Go-On, Hanox M-XL, Ostenil, and Hyalubrix) were investigated in ≤3 studies. Most studies were
high-quality and enrolled a large number of patients. In general, studies enrolled middle-aged women with mild-to-moderate
OA. Several studies did not report important study characteristics (e.g., HA products investigated, HA dose), or details on
clinical results (e.g., type of TRAE, number of events, number of patients experiencing events, timing of events).
Viscosupplementation – knee
26 studies (16 SRs, 10 RCTs) focused on knee viscosupplementation. Several outcomes such as swelling and pain at injection
site were consistently reported across studies and in agreement with other HA categories (viscosupplementation in
hands/ankles, shoulders, TMJ disorders; and scaffold), so we rated the quality of evidence as moderate. Other local responses
(e.g., flare ups, edema) and all systemic responses (e.g., cellulitis, nervous system disorders) were rated low quality due to
infrequent or rare reporting. Of 11 (42%) studies investigating systemic responses, 2 systemic skin responses (1 rash, 1
peeling of skin on hands and toes) were reported as device-related. Only 1 systemic response (GI disorders/complaints) was
reported in more than 1 study, but this outcome occurred similarly versus placebo or usual care so in both instances, the
association with HA is unclear.
Viscosupplementation – hip
4 SRs (n=2688) investigated HA products such as Durolane, Synvisc, Hyalbrix, Hyalgan, Adant. 3 SRs included placebo
injections as comparators. Quality of evidence for local responses was rated low due to the high number of patients enrolled
and similar reporting of outcomes (e.g., pain flare up, pain post-injection, hematoma) versus other categories. Systemic
responses were not investigated resulting in a rating of very low quality.
Viscosupplementation – hand and ankle
2 SRs (n=1653) investigated HA products such as Euflexxa, Durolane, and Suplasyn. 1 study each examined
viscosupplementation in hands and ankles, comparators were varied (e.g., PRP, methylprednisolone, botulinum toxin A,
prolotherapy, corticosteroids, and placebo). 1 SR of 24 studies only included 9 (37%) studies with control groups and followup
was as short as 1 month. Evidence for local responses such as swelling and pain at injection were rated low quality, while
other local responses and systemic responses (not investigated) were rated very low quality.
Viscosupplementation – shoulder
2 SRs addressed this indication in patients with glenohumeral OA and adhesive capsulitis. 1 SR only included 5 (33%) studies
with control groups. This SR pooled the AE rate for local and systemic events making it difficult to determine the AE rates
separately. This same SR did not report data for several systemic responses including serious AEs such as cancer and chest
pain, however study investigators deemed “most events” to not be product-related. The other SR reported intra-procedural
pain in 12 patients however it was unclear whether the pain was a result of the capsular distension procedure, the IAHA
injection, or both. Two local responses (musculoskeletal pain, pain at injection) were rated low quality. Other local responses
and systemic responses were rated very low quality.
Viscosupplementation – TMJ disorders
2 SRs addressing this indication reported 3 local responses including pain at injection site, ear pressure, and post-operative
discomfort. Studies reported events as occurring within 2 weeks of IAHA injections, however these responses were also
reported after other types of IA injections (CS, PRP, and PRGF) making the association with HA unclear. Pain at injection site
was reported by both SRs and in agreement with other indications for viscosupplementation (e.g., knee, shoulder, hand and
ankle), so we rated the quality of evidence as low. For other local responses and systemic responses, the quality of evidence
is very low.
Material Performance Study - Hyaluronic Acid
|
33
Cartilage scaffold
Evidence for HA scaffolds was limited to 3 studies enrolling 75 patients in 2 (66%) scaffolds of interest. Evidence for Agili-C
was lacking, and dose was NR for any study. 1 RCT reported the placement of an HA scaffold Chondrotissue in only 12
patients, while 2 nonrandomized comparative studies (led by the same investigator) reported on Hyalofast in 42 patients
(possibly including duplicate patients). These latter 2 studies both reported persistent pain, which is inconsistent with
reporting in other studies. The evidence for swelling however was reported in the RCT and was in agreement with reporting
with other HA categories (e.g., viscosupplementation-knee, and viscosupplementation-hand and ankle), so the quality of
evidence is low. For other local responses and systemic responses (no study investigating), the quality of evidence is very low.
Bone putty/filler
There were no studies that met inclusion criteria for HA bone putty/filler devices indicating an area of future research.
Safety Profile – Dermal, Facial and Eye Applications
Full Name: Hyaluronic Acid
CAS Registry Number: 9067-32-7
Safety Brief - Systematic Review Results
The systematic review included clinical and engineering literature on biocompatibility (i.e., host response and material
response) of Hyaluronic Acid used in medical devices. In addition to fundamental material biocompatibility, we focused on
specific devices known to be made of Hyaluronic Acid. The devices in Table 6 were recommended by FDA CDRH to guide ECRI
in searching this literature and ECRI’s surveillance data.
Table 6: Medical Devices Containing HA for Dermal, Facial and Eye Applications provide by FDA to Guide ECRI Searches
Regulatory Description
Product Code
Class
Aid, Surgical, Viscoelastic. (Intraocular fluid)
LZP
3
Implant, Dermal, For Aesthetic Use
LMH
3
Implant, Dermal, For Aesthetic Use In the Hands
PKY
3
Products, Contact Lens Care, Rigid Gas Permeable
MRC
2
Accessories, Soft Lens Products
LPN
2
The Safety Brief summarizes the findings of the literature search on toxicity/biocompatibility of HA for dermal, facial and eye
applications. Inclusion/exclusion criteria and quality of evidence criteria appear in Appendix A in the Appendices document.
Quality of evidence ratings reflected a combination of the quality of comparative data (study designs), quantity of evidence
(number of relevant studies), consistency of evidence, magnitude of effect, directness of evidence, and evidence for a dose
response or response over time. The search strategy appears in Appendix B2, and a flow diagram documenting
Material Performance Study - Hyaluronic Acid
|
34
inclusion/exclusion of studies appears in Appendix C2. Summary evidence tables with individual study data appear in Appendix
D2, and a reference list of studies cited in the Safety Brief appears in Appendix E.
A summary of our primary findings is shown in Table 7. We then turn to a detailed discussion of research on HA for dermal
fillers, intraocular/ophthalmic viscoelastic solution/fluid, and eye drops.
In the discussion section, please note that a statement of “no difference” or “no significant difference” between
devices/materials does not imply equivalence between devices/materials, as studies with low numbers of patients or events
often lack sufficient statistical power to detect a difference between comparators. In addition, when we cite odds ratio(s), an
odds ratio >1 means that the rate was higher in the HA group than in the non-HA group.
Table 7: Summary of Primary Findings from Systematic Review - Dermal, Facial and Eye Applications
Application
Local Host
Responses/Device Events
Quality of Evidence
(local responses)
Systemic
Responses
Quality of Evidence
(systemic responses)
Dermal Fillers
Lumpiness, tenderness,
pain, swelling, bruising,
hematoma, edema,
numbness, irregular
surface, disfiguring
surface, nodules,
granuloma, necrosis,
migration, vascular
adverse events, infection,
erythema, delayed
inflammatory reactions,
delayed-type
hypersensitivity, partial or
total vision loss, visual
changes, brain infarcts or
hemorrhage, capsular
contraction, early
resorption, embolism
Moderate
ASIA
Low
Intraocular/Ophthalmic
Viscoelastic Solution/Fluid
IOP elevations, corneal or
macular edema, ocular
hypertension,
inflammation, capsule
break, Viscoat bubbles,
layered hyphema,
choroidal effusion,
clogged tube shunt, TASS,
Descemet membrane
detachment
Moderate for IOP
elevation and corneal/
macular edema
Low for all other
responses/events
Not
evaluated
Very low
Eye Drops
Itching/stinging/irritation/
erythema/keratitis, eye
pain, eye disorders/dry
eye,
hyperemia/hemorrhage,
Moderate
Not
evaluated
Very low

Material Performance Study - Hyaluronic Acid
|
35
Application
Local Host
Responses/Device Events
Quality of Evidence
(local responses)
Systemic
Responses
Quality of Evidence
(systemic responses)
infection/viral
conjunctivitis
ASIA = autoimmune/inflammatory syndrome induced by adjuvants; IOP = intraoperative pressure; TASS = toxic anterior
segment syndrome
Dermal Fillers
The literature search identified 33 relevant human studies (6 systematic reviews (SRs),
41-46
11 randomized controlled trials
(RCTs),
47-57
4 comparative observational studies,
58-61
12 single-arm
62-73
) and 1 comparative observational animal study.
74
For
further information see Tables 1 and 2 in Appendix D.
The human studies examined over 70 different HA dermal fillers including Aesthefill, Allaxin, Bellast, Belotero, BioHyalux, a
biphasic HA filler (NASHA), Captique, CPMHA, Dermalax implant plus, Dermicol-P35, DGE, DHF-001, DIVAVIVA, Elravie,
Emervel Classic, HA (Titan) + Infrared, HA-biphasic, HA-G-monophasic, HA-monophasic, HA-P-biphasic, Hyacorp, Hylaform,
hylan B plus, Juvederm, Juvina, Koken, Macrolane, Matridex (HA and dextranomer), Matrifill, Modelis, Neuramis, Perlane, PP-
501-A/B, Prevelle SILK, Princess Volume, Puragen, Redensity II Eyes, Restylane, RHA 4, Surgiderm, Teosyal, Terafill, Uma
Jeunesse, YVOIRE volume plus, and Zyplast. Non-HA fillers examined in these studies included calcium hydroxyapatite (CaHa;
Radiesse), autologous fibroblast and keratin gel (AFKG), collagen-based fillers, Ial System Duo, polycaprolactone (PCL),
autologous fat, lipofilling, polyalkymide, poly-L-lactic acid, and acrylamide.
The 1 animal study compared the HA filler Restylane with polymethyl methacrylate (PMMA).
74
Local Responses/Device Events (human studies)
Comparisons of HA fillers to non-HA fillers: One SR
41
included 28 studies of various dermal fillers for treatment of tear trough
deformity and reported adverse events for HA and non-HA fillers. Overall complication rates were statistically significantly
different between HA and non-HA fillers (p <0.0001), but the clinical significance is unclear since the complications are minor:
HA (50.75%); calcium hydroxylapatite (CaHa, 19.95%); autologous fibroblast and keratin gel (AFKG, 11.43%); and collagen-
based filler (90%). Prolonged edema was more likely to occur among patients receiving HA injections (11.2%) than in patients
injected with the other materials (0 to 2.1%, P < 0.00001). The Tyndall effect and lumpiness were only reported in the HA
group. Thirty of 31 patients who were noted to have product migration were in the HA group, an event rate of 1.96%
(30/1585 patients) in the HA group. However, the number of patients who received AFKG or collagen-based filler was very
low, so for these fillers some complications may not have been captured simply due to the low patient numbers. Most of the
event rate comparisons were indirect (not compared within the same studies) and therefore could have been confounded by
differences in patient characteristics across different studies.
Another SR
43
included 51 studies that evaluated dermal fillers (HA and non-HA) for treatment of nasolabial folds. Overall
complication rates were 0.59 (95% CI: 0.46–0.72) for all HA fillers, 0.4 (95% CI: 0.12–0.7) for all collagen fillers, 0.82 (95%
CI: 0.69–0.93) for Mesoglow, and 0.88 (95% CI: 0.72–0.99) for IAL-systems. However, the number of studies reporting on
non-HA fillers was relatively small compared to the number of HA studies. The most common events associated with HA
injections included lumpiness (43%), tenderness (39%), swelling (32%) and bruising (28%).
One RCT
57
with 37 patients compared HA (Juvederm Ultra 4) to CaHa for hand augmentation (randomization was by hand; all
37 patients received HA in one hand and CaHa in the other). While the HA hands experienced no adverse events, six patients
experienced 11 adverse events in hands injected with CaHa. These events included edema (n = 4), pain (n = 3), impaired
movement (n = 2) and loss of sensitivity/ touch-sensitivity (number of patients not reported). Most events resolved within 2
weeks, and no serious adverse events occurred.
One prospective controlled study
60
compared HA (Juvederm Voluma) to autologous fat for treatment of temporal hollowing.
Early complications (within 2 weeks of injection) included bruising, pain and swelling which did not differ between Juvederm
and autologous fat injection sites. Juvederm had more cases of irregular surface (5/22) compared to autologous fat (1/24).
Among late complications (> 2 weeks post-injection), swelling occurred in 3/22 Juvederm sites and no autologous fat sites; it
resolved within 12 months. Subcutaneous scarring occurred in 5/24 autologous fat sites and no Juvederm sites. Abdominal

Material Performance Study - Hyaluronic Acid
|
36
pain occurred in 2 patients due to autologous fat harvest. Disfiguring surface occurred in 2/22 Juvederm sites and 1/24
autologous fat sites.
One retrospective controlled study
61
compared HA (Macrolane) to lipofilling for penile augmentation. Patients treated with
Macrolane had no complications, while 8 patients treated with lipofilling developed granulomas. In 5 patients granulomas
resolved spontaneously within 6 months with the help of massage, while 2 patients reported no relief from massage. After 20
days one patient experienced fat necrosis with progressive skin loss which was treated conservatively with weekly dressing,
and secondary healing occurred after 3 months.
Comparisons of different HA fillers: One SR
43
reported that overall complication rates did not differ between monophasic
(0.59, 95% CI: 0.36–0.8) and biphasic HA fillers (0.6, 95% CI: 0.43–0.76). Complications included redness, bruising, swelling,
pruritus, skin induration, tenderness, skin discoloration, pain, nodulus, hematoma, infection, vascular adverse events,
migration, numbness, and lumpiness. The most common complications included lumpiness, tenderness, swelling, and bruising.
Individual RCTs
48,50-52,54,56
did not report a difference in adverse event rates between different HA fillers. However, one
retrospective comparative study
59
reported that delayed-onset nodules occurred at a higher rate among patients receiving
Juvederm Volbella (1%) compared to Juvederm Vollure (0%), Juvederm Voluma (0%) and Restylane Silk (0.25%). The
nodules developed 6 to 37 weeks after the last injection.
Single-arm studies of HA fillers: One SR
44
evaluated prospective and retrospective studies of HA fillers to estimate the
incidence and prevalence of delayed inflammatory reactions and delayed-type hypersensitivity (DTH). Combined data from 35
prospective studies led to an estimated incidence of delayed inflammatory reactions of 1.1% per year, and that of possible
DTH reaction was 0.06% per year. The majority of 7 retrospective studies estimated a percentage of delayed inflammatory
reactions of less than 1% in 1 to 5.5 years. The incidence of DTH reaction cannot be determined from retrospective studies
but would likely be similar to the rate calculated from the prospective studies. Only about 5% of all reported DTH cases were
proven to be genuine DTH reactions. Two small single-arm case series
63,67
reported on delayed inflammatory reactions at HA
filler injection sites following Covid-19 exposure (3 cases) or exposure to influenza-like illness (14 cases). Most cases occurred
within 3 to 5 days of exposure for influenza, but the Covid-related cases showed a wider range (1 day to 2 weeks following
exposure either to Covid or Covid vaccine).
One large retrospective single-arm cohort study
64
evaluated the rate of delayed adverse events (DAEs) among 4500 patients
who received 9,324 treatments of HA filler (Juvederm Voluma). Forty-four patients experienced DAEs (incidence rate 0.98%
per patient, 0.47% per treatment). The most common reactions were delayed swelling and nodule formation (29 each), along
with erythema, warmth, and tenderness or pain. The median time to reaction was 4 months after the final HA injection.
Fifteen of 44 cases (34.1%) had an identifiable immunologic stimulus such as flu-like illness, infection, or dental procedure
immediately before DAE onset. Another large retrospective cohort study
68
reported that delayed-onset nodules occurred in
0.5% of Juvederm injections (23 cases out of 4,702 facial injections in 2,342 patients) with median time of onset 4 months
post-injection.
One small retrospective study
66
evaluated 11 cases of delayed granuloma formation in the orofacial region 3 to 10 years after
dermal filler orofacial injections (8 had received HA injections). All cases required surgical removal of granulomas.
One SR
45
included 26 studies reporting on 44 cases of vision loss following HA dermal filler injections. The symptoms included
partial or complete loss of vision and periocular changes including ptosis, ophthalmoplegia and exotropia; 8/44 patients had
CNS involvement in the form of brain infarcts or hemorrhage. Vision loss symptoms were almost always immediate; CNS
involvement was 7 and 9 hours after injection in 2 cases. The most common injection sites associated with visual loss were
the nose, forehead, and glabella, and the prognosis was better for patients with partial vision loss. No cases occurred
following injections in the lower face. A recent case series
62
reported on 7 cases with similar symptoms due to periorbital
vessel occlusion following HA facial injections.
Two single-arm studies reported complications related to use of Macrolane for breast enhancement. A retrospective case
series
69
of 20 patients with Macrolane-related complications presented with breast lumpiness and noted rapid, asymmetric
losses in breast volume. Cysts developed within 12 months of injection At least 85% of Macrolane filler was surgically removed
from the breasts of all patients. A prospective study
70
reported complication rates among 194 patients who received
Macrolane: minor adverse events 12.4% (24 patients), major adverse events 8.7% (17 patients), infection 0.5% (1 patient),
capsular contraction 4.6% (9 patients), early resorption 3.1% (6 patients), and removal of product 0.5% (1 patient). Most
events occurred within the first 6 months.

Material Performance Study - Hyaluronic Acid
|
37
A large retrospective case series
65
of HA fillers used for non-surgical rhinoplasty reported that the only complication was
persistent tip redness in 2% of patients that resolved spontaneously.
One prospective single-arm ultrasound study
58
of 63 female patients found alterations of the lacrimal, parotid, and
submandibular glands after filler injection (86% of patients had HA injections) that suggest subclinical inflammatory responses
to fillers. Further research would be needed to determine if ultrasound findings are clinically useful for preventing adverse
events related to dermal fillers.
Local Responses/Device Events (animal studies)
One non-randomized animal study
74
evaluated the risk of embolism and necrosis of HA (Restylane) versus PMMA (Artecoll)
injected intra-arterially in rabbit ears. With 0.1 ml injected volume, 5% of PMMA-injected ears had mild necrosis on day 7,
while HA tended to form obvious transparent emboli and 60% of HA-injected ears showed necrosis on day 7. With 0.2 ml
injected volume, 30% of PMMA-injected ears had necrosis and 100% of 0.2 ml HA-injected ears showed transparent emboli
and necrosis. HA injection led to substantially increased areas of necrosis compared to PMMA group regardless of injection
volume.
Systemic Responses
Two retrospective single-arm studies
72,73
reported on cases of patients suffering from late-onset, inflammatory, non-infectious
adverse reactions related to dermal fillers/implants that could be totally or partially considered as autoimmune/ inflammatory
syndrome induced by adjuvants (ASIA)-related disorders. The largest study (45 cases) included 7 cases who had received HA
as the only filler, and the other study (15 cases) included 2 cases where HA was the only filler (and 1 case where HA was the
second filler, following an earlier injection of silicone). Reported symptoms of HA cases in the larger study included 4 myalgia,
6 arthralgia/arthritis, 6 fatigue, 2 neurologic complaints, 2 cognitive features, 1 fever, 1 Sicca syndrome, 7 skin manifestations
(3 facial nodules), 3 evolvement into autoimmune disease. The symptoms occurred between 6 and 317 months following
exposure to dermal filler. These appear to be rare events but the rate of occurrence could not be determined because the
total number of HA-treated patients was not reported in either study.
Overall Quality of Evidence
Several SRs and RCTs provided evidence concerning local responses/adverse events associated with HA dermal fillers, so the
overall quality of evidence for local responses/events is moderate. Two small retrospective single-arm case series reported
evidence of rare systemic responses in the form of autoimmune/inflammatory syndrome associated with HA fillers; the quality
of evidence for systemic responses is therefore low.
Intraocular/Ophthalmic Viscoelastic Solution/Fluid
The literature search identified 10 human studies (1 SR,
75
2 RCTs,
76,77
3 observational comparative studies,
78-80
and 4 single-
arm studies
81-84
). For further information see Table 3 in Appendix D.
The human studies examined ophthalmic viscoelastic devices (OVDs) including Healon, Healon5, Healon GV, 2% HPMC,
Viscoat, OcuCoat, Provisc, Restylane-L, SKGEL, Soft Shell, DisCoVisc, Duovisc, and Twinvisc.
Local Host Responses (human studies)
All studies evaluated local responses/device events related to various ophthalmic viscoelastic (HA) solutions in human
subjects.
Four studies compared intraoperative pressure (IOP) elevations following eye surgery and injection of HA solutions to other
HA solutions or no HA solution. One SR
75
reported that Healon, Viscoat, Provisc, and Soft Shell were associated with
statistically significant increases in IOP at 1 day post-surgery while other HA solutions (Healon GV, Healon5, 2%HPMC,
OcuCoat, and Viscoat + Provisc showed either non-statistically significant increases or decreases in IOP. At 1-week follow-up,
most HA solutions were associated with non-statistically significant reductions in IOP; only Healon GV and Healon5 were
associated with statistically significant decreases in IOP. The remaining 3 studies (2 RCTs
76,77
and 1 retrospective comparative
study
78
) showed no statistically significant difference in post-operative IOP levels between different HA solutions
76,77
or
between HA and no HA.
78
These 3 studies did not evaluate Healon GV or Healon5. Overall, IOP tended to peak at 1 day post-
surgery and decrease afterward.

Material Performance Study - Hyaluronic Acid
|
38
Three studies (1 SR
75
and 2 RCTs
76,77
) reported cases of corneal and/or macular edema. These were rare events (the SR
reported rates of corneal edema ranging from 0.6% to 2.5% across 4 studies, while macular edema rates were 0.6% and
0.9% in 2 studies, respectively) and there was no statistically significant difference in edema rates between groups of patients
receiving different HA solutions.
One RCT
76
reported ocular hypertension in 12.6% of patients who received Twinvisc and 17.6% of patients who received
Duovisc. This study also reported 1 case each of inflammation (0.9%), capsule break (0.9%) and Viscoat bubbles (0.9%),
respectively.
One retrospective comparative study
78
reported that 20 of 95 (21.1%) of patients with HA fill (Provisc) had a layered hyphema
compared with 2 of 45 (4.4%) in the control group (no HA fill) at 1 week post-surgery (P=0.01). One patient (1.0%) in the HA
group and 1 patient (2.2%) in the non-HA group required drainage of choroidal effusions within the first postoperative month.
Other complications included a retinal detachment in a patient without HA fill and a clogged tube shunt that occurred in a
patient who received HA fill.
One prospective observational study
80
compared non-penetrating very deep sclerectomy (NPVDS) with the use of HA implant
(SKGEL) to trabeculectomy (TB) in patients with medically uncontrolled glaucoma. The overall complication rate was higher in
the TB group (79.5% vs 35.9%, p = 0.00011); whether the lower rate in the NPVDS group is related to HA or the sclerectomy
procedure (or both) is unclear.
One retrospective single-arm study
82
reported on 34 patients who developed toxic anterior segment syndrome (TASS) after
routine cataract surgery. The authors identified the source of TASS as HA (2.0% or 1.4%) derived from rooster comb; when
they substituted with OVDs derived from bacterial fermentation no further cases of TASS were observed.
One retrospective single-arm study
83
with 115 patients (162 eyes) who received canaloplasty for glaucoma reported that 12
patients (7.4%) developed Descemet membrane detachment (DMD) with or without intracorneal hemorrhage. All cases
resolved in 4 to 10 weeks (3 patients required additional surgery to resolve the DMD). Although the cause of DMD was
unclear, the authors noted that “DMD could be related to excessive amounts of Healon GV injection into the Schlemm canal
during the viscodilation portion of the surgery.”
One retrospective study
79
compared a viscoelastic device with brilliant blue G (Visco-BBG) versus balanced salt solution with
BBG (BSS-BBG) in patients who were receiving par plana vitrectomy (PPV) combined with internal limiting membrane (ILM)
peeling. The authors reported no complications in the Visco-BBG group and only 1 complication (retinal perforation) in the
BSS-BGG group.
Two single-arm observational studies
81,84
reported only complications that were considered to be unrelated to HA solutions.
Systemic Responses
No studies reported whether any systemic responses occurred.
Overall Quality of Evidence
The evidence regarding IOP came from 1 SR and 3 comparative studies (2 RCTs) and was of moderate quality, supporting an
initial increase followed by decrease in IOP after 1 day post-surgery. Some HA solutions (Healon GV, Healon5) appeared to
increase IOP less and decrease IOP sooner than others. Evidence that corneal and macular edema are rare events and have
similar rates among different HA solutions was also moderate quality. Evidence for other outcomes were reported in fewer
studies (mostly single studies, mostly observational) and the quality of evidence is low. Because no studies reported systemic
responses, the quality of evidence regarding systemic responses is very low.
Eye Drops
The literature search identified 14 human RCTs. For further information see Table 4 in Appendix D. The studies examined HA-
containing eye drops including Hylo Confort Plus/HydoGel, Lacure, Restasis, Tearin, Vislub®, Vismed®, Vismed® Multi, HA-
trehalose (Thealoz Duo®/Thealose®). These products contained 0.1% to 0.2% HA.
Local Host Responses (human studies)
All 14 studies evaluated local responses/device events related to procedures utilizing HA eye drops in human subjects.

Material Performance Study - Hyaluronic Acid
|
39
Eight studies
85-92
addressed general adverse events or complications related to HA. Four studies
85,88,90,92
reported no difference
in adverse events between HA and a control or another treatment group. One study
89
found statistically significantly fewer
adverse events when compared to another treatment group (p=0.0186). Three studies
86,87,91
found less than 10% of patients
experienced adverse events where both groups contained HA.
Seven studies
85,93-98
addressed itching/stinging/irritation/pruritus. Two studies
85,98
showed a decrease in
itching/stinging/irritation/erythema/keratitis when HA was compared to another treatment or a control. Four studies
93,95-97
showed no statistically significant difference in itching/stinging/irritation/erythema/keratitis when HA was compared to another
treatment or a control. One study
94
showed less than 2% of patients experience itching/stinging/irritation/erythema/keratitis
where both groups contained HA.
Six studies
85,94-98
addressed eye pain. Four studies
85,95,97,98
showed no statistically significant difference in pain when HA was
compared to another treatment or a control. One study
94
showed less than 2% of patients experience pain where both groups
contained HA. One study
96
showed an increase in pain when HA was compared to another treatment or a control.
Five studies
89,93,94,97,98
addressed eye disorders/dry eye. Two studies
93,94
showed 12% or fewer patients experienced eye
disorders/dry eye where both groups contained HA. One study
97
showed no statistically significant difference in eye
disorders/dry eye when HA was compared to another treatment or a control. Two studies
89,98
showed a decrease in eye
disorders/dry eye when HA was compared to another treatment or a control.
Four studies
95-98
addressed hyperemia/hemorrhage. All 4 studies
95-98
showed no statistically significant difference in
hyperemia/hemorrhage when HA was compared to another treatment or a control.
Three studies
94,95,98
addressed infection/viral conjunctivitis. Two studies
95,98
showed no statistically significant difference in
infection/viral conjunctivitis when HA was compared to another treatment or a control. One study
94
showed less than 2% of
patients experience infection/viral conjunctivitis where both groups contained HA.
Systemic Responses
No studies reported whether any systemic responses occurred.
Overall Quality of Evidence
The evidence base for human studies is large and includes 14 RCTs. The findings were mostly consistent in showing that HA
did not play a large role in local host response or may have reduced the incidence of local host response. Some of the studies
did not address the effects HA may have locally, as both experimental groups contained HA. Therefore, the quality of evidence
for local host response is moderate
.
Because no studies reported on systemic responses, the quality of evidence for systemic
responses is very low.
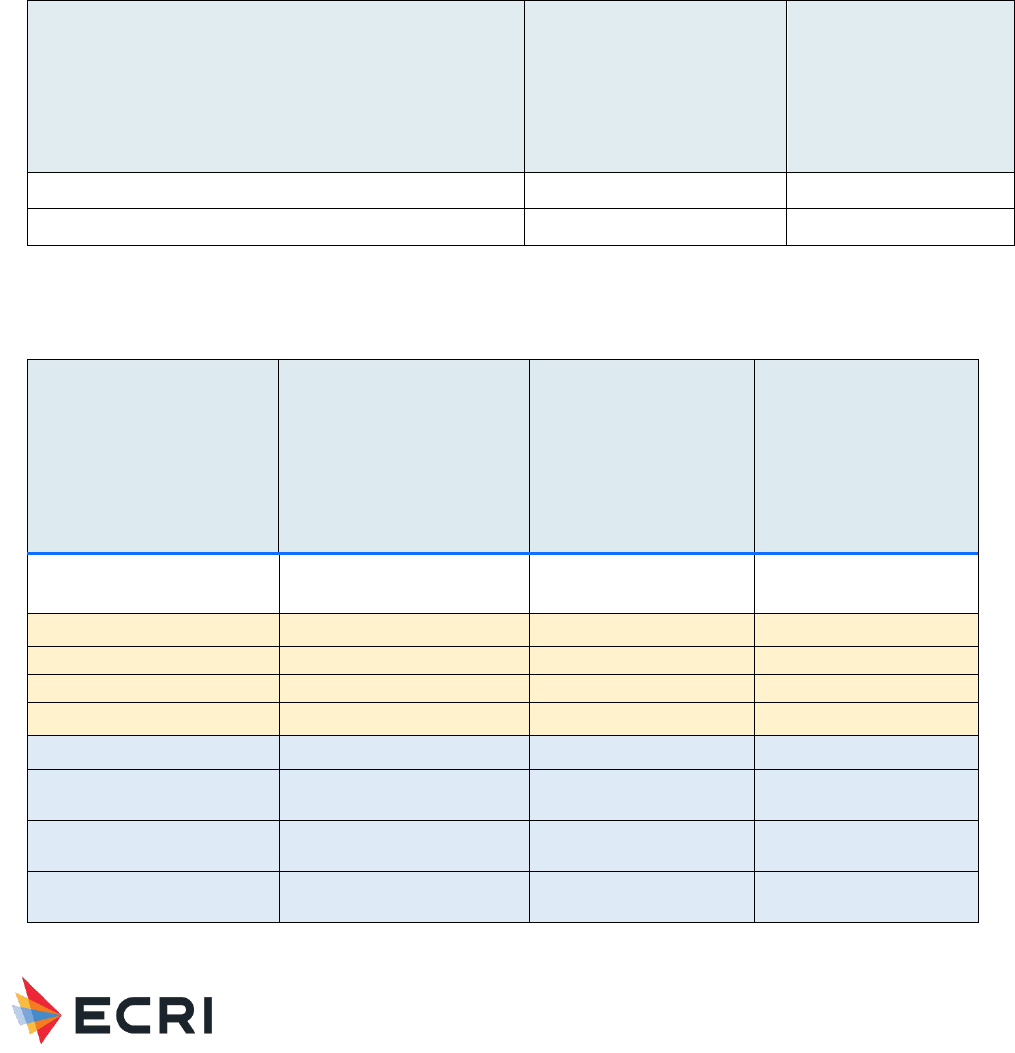
Material Performance Study - Hyaluronic Acid
|
40
ECRI Surveillance Data
Patient Safety Organization
Search Results: ECRI PSO identified a total of 6 reports that involved HA materials that occurred between July 2016 and
August 2021. One of these reports is pertinent to the topic of this report and it involved a hemorrhage/hematoma associated
with a dermal implant. Table 8 and Table 9 outline the PSO event report complications and harm scores, respectively.
All individual PSO event reports are redacted and included in Appendix F.
Table 8: Complications in HA-related PSO Event Reports - Dermal, Facial and Eye Applications
Complication
Dermal implant
Total
Hemorrhage/Hematoma
1
1
Total
1
1
Table 9: Harm Score Associated with HA-related PSO Event Reports - Dermal, Facial, and Eye Applications
Harm Scores (NCC-MERP) Harm Scores (NCC-MERP)
Dermal implant
Total
A
No Error
B1
Error, No Harm
B2
Error, No Harm
C
Error, No Harm
D
Error, No Harm
E
Error, Harm
F
Error, Harm
G
Error, Harm
H
Error, Harm
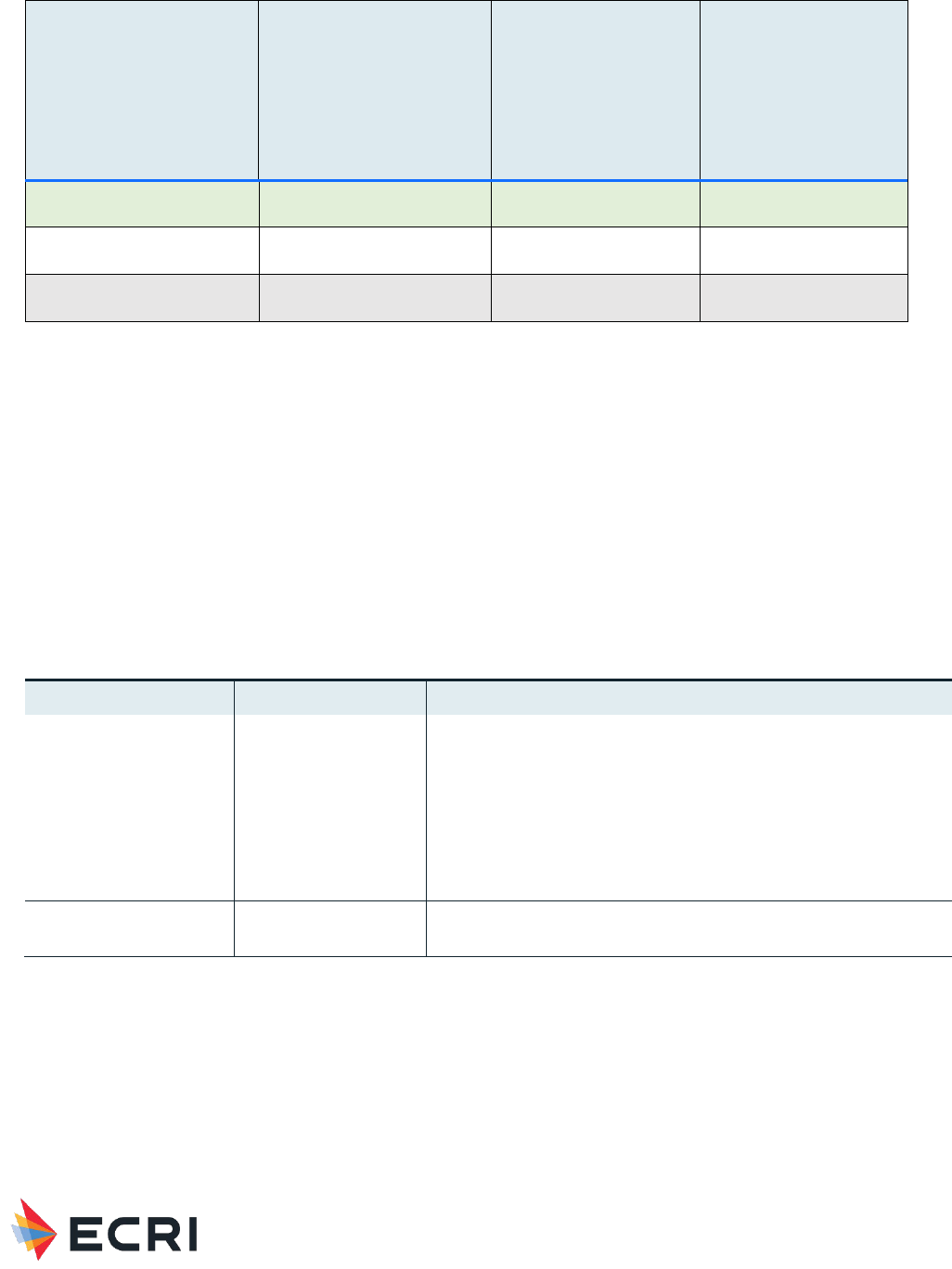
Material Performance Study - Hyaluronic Acid
|
41
Harm Scores (NCC-MERP) Harm Scores (NCC-MERP)
Dermal implant
Total
I
Error, Death
NULL*
1
Total
1 1
*Harm score was not reported
Accident Investigations
Search Results: The search returned zero accident investigations associated with HA for dermal, facial and eye applications.
ECRI Problem Reports
Search Results: The search returned zero reports submitted by ECRI members.
Healthcare Technology Alerts
Search Results: The search returned 7 manufacturer issued alerts describing problems with technique, updated IFU,
discontinued use, clogged equipment, difficulty removing from body, contamination, leakage, and compromised sterility,
summarized in Table 10.
Table 10: Summary of Regulatory and Manufacturer Alerts - Dermal, Facial and Eye Applications
Device Type
# Alerts
Reported Problem
LZP (Aid, Surgical
Viscoelastic) –
Intraocular Fluid
5 manufacturer issued
• Product is difficult to remove from the eye, increasing post-op
intraocular pressure
• Clogged phacoemulsification equipment can lead to ocular
injury
• Product may contain microscopic glass particles
• Cannulae may leak viscoelastic material
• Inadequately sealed syringes compromise sterility
• Manufacturer reminds users of proper technique
Dermal Implant
2 manufacturer issued
• Updated IFU
• Discontinued use for breast augmentation
Potential Gaps
ECRI surveillance searches reflect mostly acute patient incidents that involved medical devices made of Hyaluronic Acid. Areas
of particular concern involve incidents that result in direct tissue exposure to the material if there is moderate to high-quality
evidence of acute or systemic reaction to this exposure, as determined by the systematic review. Topics with very low or low
quality of evidence represent areas of potential gaps in the literature. If the literature revealed areas of new concern (e.g.,
systemic response to long-duration contact) and there is little supporting evidence, these are considered gaps.
Across all device categories, the overall quality of evidence was low to very low for potential systemic responses.

Material Performance Study - Hyaluronic Acid
|
42
For intraocular/ophthalmic/viscoelastic solution/fluids, other than IOP elevation and corneal/macular edema (moderate quality
of evidence), all identified local host responses (e.g., ocular tension, inflammation) were of low quality of evidence.
There were no studies that addressed any particular cellular or molecular mechanisms for systemic manifestations.
Additionally, no studies addressed patient-related or material-related factors that could predict the likelihood and/or severity of
immunological/systemic responses.
Material Performance Study - Hyaluronic Acid
|
43
Safety Profile – Adhesion Barrier and Bulking Agent
Applications
Full Name: Hyaluronic Acid
CAS Registry Number: 924474
Safety Brief - Systematic Review Results
The systematic review included clinical and engineering literature on biocompatibility (i.e., host response and material
response) of Hyaluronic Acid used in medical devices. In addition to fundamental material biocompatibility, we focused on
specific devices known to be made of Hyaluronic Acid. The devices in Table 11 were recommended by FDA CDRH to guide
ECRI in searching this literature and ECRI’s surveillance data.
Table 11: Medical Devices Containing HA for Adhesion Barrier and Bulking Agent Applications provided by FDA to Guide ECRI
Searches
Regulatory Description
Product Code
Class
Balloon, Epistaxis
EMX
1
Mesh, Surgical, Polymeric
FTL
2
Mesh, Surgical
FTM
2
Cuff, Nerve
JXI
2
Polymer, Ent Synthetic-Polyamide (Mesh or Foil
Material
KHJ
2
Implant, Dermal, for Aesthetic Use
LMH
3
Agent, Bulking, Injectable for Gastro-Urology Use
LNM
3
Splint, Intranasal Septal
LYA
1
Barrier, Absorbable, Adhesion
MCN
3
Dressing, Wound and Burn, Hydrogel with Drug and/or
Biologic
MGQ
Unclassified
System, Vocal Cord Medialization
MIX
2
Polymer, Ear, Nose and Throat, Synthetic, Absorbable
NHB
2
The Safety Brief summarizes the findings of the literature search on toxicity/biocompatibility of Hyaluronic Acid.
Inclusion/exclusion criteria and quality of evidence criteria appear in Appendix A in the Appendices below. Quality of evidence
ratings reflected a combination of the quality of comparative data (study designs), quantity of evidence (number of relevant
studies), consistency of evidence, magnitude of effect, directness of evidence, and evidence for a dose response or response
over time. The search strategy appears in Appendix B3, and a flow diagram documenting inclusion/exclusion of studies
appears in Appendix C3. Summary evidence tables with individual study data appear in Appendix D3, and a reference list of
studies cited in the Safety Brief appears in Appendix E.
Material Performance Study - Hyaluronic Acid
|
44
Hyaluronic acid (HA) is a natural polymer found in the extracellular matrix and in high concentrations in the skin and synovial
fluid. HA suppresses immune responses and inflammation and aids wound healing. Based on these properties HA has been
incorporated into a variety of medical products designed to reduce postsurgical inflammation.
99,100
A summary of our primary findings is shown in Table 12. Even though the table mentions local host “responses” as well as
systemic “responses”, the events listed were not necessarily caused by the material. Only a few of these events were
statistically significantly more likely with HA than without HA. Some listed events were actually statistically significantly less
likely with vs without HA (in which case we write the word “less” next to the event). After the table, we present a detailed
discussion of research on HA as a material as well as research on the various device categories. For three additional device
categories, our searches identified no evidence (anti-adhesion protective oral gels, intravesical agents, and protective topical
agents).
Table 12: Summary of Primary Findings from Systematic Review - Adhesion Barrier and Bulking Agent Applications
Application
Local Host Responses/Device Events
Quality of
Evidence
(local
responses)
Systemic Responses
Quality of
Evidence
(systemic
responses)
Hyaluronic Acid as a
material (1 human
study, no animal
studies)
Hematuria, nausea
Low
Urticaria
Low
Adhesion barrier (34
human studies, no
animal studies)
Abdominal abscess, Acute pyelonephritis,
Anastomotic fistula, Anastomotic leakage,
Blood transfusion, Bowel injury, Cardiovascular
complications, Changes in menstrual pattern,
Hyperthermia, Ileus, Incision site abscess,
Intestinal fistulas , Intra-abdominal abscess,
Less Adhesion bowel obstruction, Less Post-
operative intestinal obstruction , Paralytic
ileus, Parietal abscess, Pelvic abscess,
Peritonitis, Pleural effusion, Pneumonia,
Postoperative biloma, Postoperative right
portal vein embolization, Respiratory
complications, Septic shock, Surgical-site
infection, Suture failure, Transfusion, Urinary
tract infection, Uterine dehiscence, Wound
infection
High
Alanine
aminotransferase,
Albumin, Aspartate
aminotransferase,
Bilirubin, Blood urea
nitrogen, C-reactive
protein, Creatinine,
Fever, Glucose,
Hemoglobin, Liver
enzymes, Liver
function, Renal
function, WBC
count, White blood
cell count
High
Anti-adhesion: Nasal
packaging (5 human
studies, no animal
studies)
No events
Moderate
None investigated
Very Low
Barrier gel for oral
lesions (3 human
studies, no animal
studies)
Less inflammation
Moderate
None investigated
Very Low
Material Performance Study - Hyaluronic Acid
|
45
Application
Local Host Responses/Device Events
Quality of
Evidence
(local
responses)
Systemic Responses
Quality of
Evidence
(systemic
responses)
Bulking agents (3
human studies, no
animal studies)
Urinary tract infection proctalgia, rectal
hemorrhage, diarrhea, injection site
complications, injection site sterile abscess,
injection site mass, pseudo-cyst formation
Moderate
Fever
Moderate
Intravesical agents
for bladder pain
syndrome/interstitial
cystitis therapy (2
human studies, no
animal studies)
Less urinary tract infection
Low
None investigated
Very Low
Organ spacer (2
human studies, no
animal studies)
Injection site pain, lower abdomen pain,
hematuria
Low
Asthenia
Low
Protective topical
agent for
esophageal and
gastric lesions in
GERD (1 human
study, no animal
studies)
Cough, rhinitis, throat irritation
Low
Nervous system
event (unspecified),
vertigo,
hypertension
Low
Vocal cord/fold
medialization (1
human study, no
animal studies)
Hematoma, edema of the aryteno-epiglottic
fold and false vocal fold
Low
None investigated
Very Low
Hyaluronic Acid as a Material
One human study (one randomized controlled trial [RCT]), no animal studies. For more information, see Table 1 in Appendix
D.
Local Responses (human studies)
One RCT of men (n = 49) undergoing radiotherapy for prostate cancer assessed using HA and chondroitin sulfate instilled into
the bladder to prevent radiation-induced cystitis.
101
Patients also received oral HA and chondroitin sulfate. Control patients
received no treatment. Mild to moderate drug-related adverse events were reported in 7 HA and chondroitin sulfate treated
patients. Only 2 local adverse events were described, hematuria (blood in the urine) and nausea. Four of the seven adverse
events were not described. There were no serious local drug-related adverse events or treatment-related withdrawals. No
local adverse events were reported in control patients.
Because treated patients received HA as well as chondroitin sulfate, one cannot determine whether the reported adverse
events were due to HA or chondroitin sulfate or other factors.
Systemic Responses
In the same RCT, only 1 systemic adverse event was described: a case of urticaria (skin rash due to an allergic reaction) in
the treated group. There were no serious systemic drug-related adverse events or treatment-related withdrawals. As with
local events, the study design does not permit a determination of the cause of this event.

Material Performance Study - Hyaluronic Acid
|
46
Overall Quality of Evidence
The quality of the evidence is low for local responses and for systemic responses since the evidence comes from only one RCT
with confounding factors that included multiple additional compounds administered to the treated patients and an unblinded
control group that did not receive a placebo.
Adhesion barrier
34 human studies (14 SRs, 20 RCTs, no animal studies). For more information, see Table 2 in Appendix D.
Local Responses/Device Events (human studies)
Below, we discuss the evidence in five categories:
• Seprafilm (HA and carboxymethylcellulose)
• Sepraspray (HA and carboxymethylcellulose)
• Guardix-sol (HA + CMC gel)
• MateRegen (HA gel)
• Other HA gels
Seprafilm (HA and carboxymethylcellulose):
One SR examined SSI rate, anastomotic leak, ileus, intra‑abdominal abscess, and small bowel obstruction in RCTs using HA
and carboxymethylcellulose (CMC) barrier (Seprafilm) versus no treatment (control) during abdominal surgery.
102
The SR
included 13 RCTs with 3,665 patients, of which 1,800 were in the Seprafilm group. Use of Seprafilm was associated with a
significantly higher risk of anastomotic leak (3.1%) compared to control (1.6%), but a significantly lower incidence of small
bowel obstruction (3.0%) compared to control (5.9%).
One SR examined complications in clinical studies using HA+CMC barrier (Seprafilm) versus control on rates of postoperative
small bowel obstruction.
103
The SR included 4 RCTs and 5 clinical studies with 4,351 patients, of which 2,123 were in the
Seprafilm group. No complications were related to Seprafilm. The SR reported “the risk of postoperative small bowel
obstruction can be significantly decreased by the application of Seprafilm.”
One SR examined postoperative complications including intra-abdominal infection, wound infection, anastomotic leakage, and
postoperative hospital stay in clinical studies using HA+CMC barrier (Seprafilm) versus control after gastrointestinal neoplasms
surgery.
104
The SR included 5 RCTs and 5 clinical studies with 2,937 patients, of which 1,334 were in the Seprafilm group. The
incidence of postoperative intestinal obstruction was significantly lower in the Seprafilm group than the control (risk ratio,
0.52; 95% CI, 0.38–0.70; p<0.0001). Combined postoperative complications (intra-abdominal infection, wound infection,
anastomotic leakage, and postoperative hospital stay) were also significantly lower in the Seprafilm group than the control
(risk ratio 0.77; 95% CI, 0.61–0.97; p = .03). However, separate analysis of each postoperative complication did not find a
significant difference.
One SR examined complications in RCTs using HA+CMC barrier (Seprafilm) versus control after gastrointestinal surgery.
105
The SR included 5 RCTs with 2,177 patients. The SR reported “As regards adverse effects, the use of Seprafilm has not been
shown to result in a significant increase in morbidity, but…when Seprafilm was placed in direct contact with intestinal
anastomoses, a significant increase in the rate of intestinal fistulas and their secondary morbidity was observed.”
One RCT of 95 metastatic colorectal cancer patients requiring 2-stage hepatectomy compared HA+CMC barrier (Seprafilm)
with no HA control.
106
There was no significant difference in complication rates between Seprafilm and control groups. Major
complications included postoperative biloma that required percutaneous drainage and postoperative right portal vein
embolization. Minor complications included urinary tract infections, minor pleural effusion, blood transfusion and parietal
abscess.
One RCT of 345 patients undergoing elective colectomy compared HA+CMC barrier (Seprafilm) with no HA control.
107
No
complications were related specifically to Seprafilm. Surgery-related complications seen equally in both groups included
surgical-site infection, suture failure, and respiratory and cardiovascular complications.
One RCT of 753 females undergoing caesarean delivery compared HA+CMC barrier (Seprafilm) with no HA control.
108
No
complications were related specifically to Seprafilm. Surgery-related complications seen equally in both groups included bowel
injury, intraoperative transfusion, and uterine dehiscence.

Material Performance Study - Hyaluronic Acid
|
47
One RCT of 488 patients undergoing colorectal surgery compared HA+CMC barrier (Seprafilm), HA+CMC gel (Guardix), and
no HA control groups.
109
No complications were specifically related to Seprafilm or Guardix. Surgery-related complications
seen equally in each group included pneumonia, surgical-site infection, anastomosis leakage, and intra-abdominal abscess.
Sepraspray (HA + CMC):
One RCT of 209 patients undergoing laparoscopic colorectal and/or small bowel surgery compared HA+CMC powder
(Sepraspray Adhesion Barrier) with no HA control (n=104).
110
The Sepraspray group had significantly higher rates of adverse
events (62.9%) and serious adverse events (27.6%) compared to control. Adverse events included hyperthermia (HA 5.7%,
Control 2.9%), incision site abscess (HA 4.8%, Control 2.9%), urinary tract infection (HA 4.8%, Control 1.0%), and
anastomotic fistula (HA 3.8%, Control 3.8%). Serious adverse events included pelvic abscess (HA 4.8%, Control 1.9%),
abdominal abscess (HA 3.8%, Control 0%), septic shock (HA 1.0%, Control 1.9%), peritonitis (HA 1.9%, Control 2.9%), ileus
(HA 2.9%, Control 0%), and anastomotic fistula (HA 2.9%, Control 3.8%).
One RCT of 41 females undergoing laparoscopic myomectomy compared HA+CMC powder (Sepraspray Adhesion Barrier) with
no HA control.
111
No adverse events were specifically related to Sepraspray. Adverse events occurred in both groups but were
not defined. The authors reported that surgical-site infections, intra-abdominal abscesses, and deep vein thrombosis did not
occur in either group.
Guardix-sol (HA + CMC gel):
One RCT of 76 patients undergoing laparoscopic radical cystectomy compared HA+CMC gel (Guardix-sol) with no HA
control.
112
Complication rates did not differ significantly in the Guardix-sol and control groups, “except for adhesive bowel
obstruction, which occurred in zero and six patients, respectively (p = 0.025).” Reported complications included transfusion
(HA 26.3%, Control 34.2%), paralytic ileus (HA 15.8%, Control 10.5%), wound disruption (HA 2.6%, Control 7.9%), and
acute pyelonephritis (HA 5.3%, Control 7.9%).
One RCT of 43 males undergoing surgery for chronic epididymitis compared HA+CMC gel (Guardix-sol) with no HA control.
113
No complications occurred in either group (specifically looked for wound infection, wound hematoma, wound deadhesion,
hematoma, cord injury, and testicular injury).
MateRegen (HA gel):
One RCT of 306 females with moderate to severe intrauterine adhesions (IUAs) undergoing operative hysteroscopy followed
by embryo transfer compared intrauterine injection of HA gel (MateRegen) with no HA control.
114
No adverse events were
observed in either group (authors did not specify what events they expected).
One RCT of 145 females with moderate to severe IUAs compared intrauterine injection of HA gel (MateRegen) with no HA
control.
115
No surgical complications were reported in either group. After surgery, changes in menstrual pattern in the
treatment group (87.7%, 107/122) were similar to the control group (76.4%, 97/123).
One RCT of 274 females undergoing dilation and curettage compared HA gel (MateRegen) with no HA control.
116
The authors
noted that “no serious adverse events were observed during the study period, and there were no prolonged hospitalizations or
reoperations owing to adverse events” in either group.
One RCT of 48 females undergoing curettage compared HA gel (MateRegen) with no HA control.
117
No complications or
adverse events were related to MateRegen. No postoperative infections, prolong hospitalizations, or reoperations occurred in
either group.
Other HA gels:
One RCT of 196 females undergoing laparoscopic surgery compared HA gel (HyaRegen NCH) with saline placebo.
118
No
adverse events were related to HyaRegen NCH. The authors reported that adverse events were comparable between groups,
mild and spontaneously resolved. No serious adverse events were observed in either group. The authors did not indicate what
adverse events were expected.
One SR examined adverse events and abdominal complaints in RCTs using HA gel (Hyalobarrier Gel) versus control after
endoscopic gynecologic surgery.
119
The SR included 5 RCTs with 335 females, of which 167 were in the Hyalobarrier Gel
group. No adverse events were related to Hyalobarrier Gel. In gel-treated patients, two reported nausea and one reported
vomiting. In control patients, one reported nausea.

Material Performance Study - Hyaluronic Acid
|
48
One SR examined pain in clinical studies using HA gel (Hyalobarrier Gel) versus control after laparoscopic gynecologic
surgery.
1
The SR included 1 RCT with 43 females, of which 25 were in the Hyalobarrier Gel group. The SR reported “Full
conditioning with Hyalobarrier Gel placement resulted in decreased pain scores (p < .001) and faster recovery (p < .0001)
compared with standard laparoscopy.”
One SR examined adverse events and complications in RCTs using HA and HA+CMC barriers versus control after laparoscopic
myomectomy (surgical procedure to remove uterine fibroids).
120
The SR included 8 RCTs with 748 patients, of which 392 were
in the HA group. No serious adverse events or complications occurred in the included studies. The authors did not state what
adverse events or complications they expected.
One SR examined complications in RCTs using HA and HA+CMC barriers versus control after gynecologic surgery.
121
The SR
included 7 RCTs with 748 females. No complications were related to HA barriers.
One SR examined complications in clinical studies using HA and HA+CMC gels versus control after operative hysteroscopy.
122
The SR included 3 clinical studies with 262 females, of which 130 were in the HA groups. No complications were related to HA
gels.
One SR, a Cochrane Review) examined adverse outcomes and pelvic pain in two SRs of RCTs comparing HA+CMC barrier
versus control for adhesion prevention after gynecologic surgery.
123
The SR included 47 RCTs with between 59 and 127
females in HA+CMC and control groups. The available studies reported no adverse outcomes or pelvic pain related to
HA+CMC barrier, but adverse event reporting occurred in only half of the studies.
One SR examined complications in RCTs using HA gel versus control after miscarriage.
124
The SR included 4 RCTs with 625
females, of which 290 were in the HA group. No complications were related to HA gel.
One SR examined complications in RCTs using HA gel versus control after intrauterine operation.
125
The SR included 7 RCTs
with 952 females, of which 455 were in the HA group. No complications were related to HA gel. The authors reported that
none of the included studies reported HA or surgery related complications, including hemorrhage, perforation or cervical
laceration.
One SR examined complications in RCTs using HA gel versus control after hysteroscopic adhesiolysis.
126
The SR included 2
RCTs and 4 clinical studies with 394 females, of which 176 were in the HA group. No complications occurred in the HA gel
group.
One SR examined complications in RCTs using HA gel versus control after gynecologic surgery.
127
The SR included 6 RCTs
with 564 females, of which 286 were in the HA group. Only one RCT reported HA group complications, which were “patient
discomfort and uterine perforation, with no statistically significant differences between the 2 [HA and control] groups.”
One RCT of 65 females undergoing uterine septum resection compared intrauterine injection of HA with no HA control.
128
No
complications, such as allergic reactions, occurred in the HA group. Menstrual patterns were not affected in either group.
One RCT of 143 females undergoing dilation and curettage compared HA gel with no HA control.
129
No complications occurred
in the HA group. Menstrual patterns and pregnancy rates were not affected in either group.
One RCT of 149 females undergoing dilation and curettage compared HA gel with no HA control.
130
No complications were
specifically related to HA gel. Cervix laceration was experienced by one patient in the HA group (1.3%) and no patients in the
control group (p=1.00). Three patients in the HA group had excessive bleeding (3.9%) compared to one patient in the control
group (1.4%) (p=0.62). Two patients in the HA group had postoperative infections (2.6%) compared to one patient in the
control group (1.4%) (p=1.00). Four patients in the HA group had postoperative pain (5.2%) compared to three patients in
the control group (4.2%) (p=1.00). Uterus perforation was experienced by one patient in the HA group (1.3%) and no
patients in the control group (p=1.00).
One RCT of 124 females undergoing laparoscopic surgery for deep infiltrating endometriosis compared HA gel with sterile
saline placebo.
131
No complications were related to HA gel. Patient pain was significantly reduced in the HA gel group after six
months. HA treatment significantly reduced dysmenorrhea (painful menstruation), dyschezia (difficult or painful defecation),
and dyspareunia (painful intercourse) at 3 and 6 months compared with control patients.
One RCT of 87 patients undergoing colorectal surgery compared HA gel, chitosan, and control groups and reported short-term
(peristomal cutaneous infection, intra-abdominal hemorrhage, and abscess) and long-term (intestinal obstruction and
anastomotic leakage) postoperative complications.
132
No complications were statistically significantly more likely in the HA gel

Material Performance Study - Hyaluronic Acid
|
49
group. Short-term (30-day) complications were not seen in any patients. Long-term postoperative complications were similar
among groups (10.5% of HA gel patients and 17.9% of control patients).
One RCT of 89 females undergoing operative hysteroscopy for intrauterine adhesions compared HA gel, intrauterine device
(IUD), and HA gel+IUD groups.
133
No complications occurred in the HA gel group. None of the patients experienced excessive
bleeding, infection, or other complications (not specified by the authors).
Systemic Responses
One SR examined systemic complications reported in 2 RCTs and 1 comparison study using Seprafilm (HA containing
membrane) to prevent intestinal obstruction after gastrointestinal neoplasms surgery.
104
The SR reported aspartate
aminotransferase, alanine aminotransferase (serum indicators of liver function), and blood urea nitrogen (indicator of kidney
function) were not significantly different from controls five- and seven-days post-surgery. However, serum creatinine (another
indicator of kidney function) was significantly higher in Seprafilm patients at 5 days but not at 7 days. The authors noted “that
Seprafilm did not cause acute postoperative inflammation in short term, and had almost no effect on the liver and renal
function of the patients.”
One SR examined systemic complications in 5 RCTs comparing cross-linked HA gel with no treatment in patients undergoing
endoscopic gynecological surgery.
119
Fever was reported in one of 167 HA patients and 2 of 168 control patients.
One RCT of 753 women undergoing caesarean delivery compared Seprafilm with no Seprafilm control.
108
Fever was reported
in similar numbers of patients (4.5% Seprafilm, 3.0% control, risk ratio = 1.5 (95% CI of 0.7 to 3.3)).
One RCT of 196 women undergoing laparoscopic surgery compared a crosslinked hyaluronan gel with saline placebo.
118
The
RCT examined blood chemistry (C-reactive protein, WBC count, hemoglobin, liver enzymes, albumin, blood urea nitrogen,
creatinine, bilirubin, glucose, etc.) at 3 days, 30 days, and 9 weeks and found no clinically significant changes from baseline in
the hyaluronan gel patients. Control patients had clinically significant elevations in white blood cell count and blood glucose
from baseline at 9 weeks.
One RCT of 87 patients undergoing colorectal surgery compared HA gel with chitosan or no treatment.
132
White blood cell
count, liver function, and renal function were comparable across groups at 2 weeks post-surgery.
Overall Quality of Evidence
The quality of evidence is high for local responses since the evidence comes from multiple SRs and RCTs reporting consistent
results that there are few adverse events or complications related to HA-containing products. The quality of evidence is high
for a lack of systemic responses since the evidence is from SRs with multiple RCTs and three individual RCTs.
Anti-adhesion: Nasal packaging
Five human studies (two SRs, three RCTs), no animal studies. For more information, see Table 3 in Appendix D.
Local Host Responses (human studies)
One SR examined complications reported in RCTs using Sepragel or MeroGel, both contain HA, as nasal packing after
endoscopic sinus surgery.
134
The SR included four RCTs with 352 patients. According to the SRs, no adverse events or
complications were reported in two of the included RCTs, and SR authors did not describe the other two RCTs results. No
specifics were provided on what adverse events may have been expected.
One SR examined complications in studies evaluating endoscopic sinus surgery.
135
The SR included 13 studies with 501
patients (5 double-blind RCTs, 5 single-blind RCTs, and 3 prospective comparisons) that used HA as an absorbable packing, a
non-absorbable packing impregnated with HA, or an HA spray. The SR reported “It is clear that hyaluronic acid in all
preparations is safe and well-tolerated by patients, as evidenced by only one adverse event not related directly to hyaluronic
acid, and the overall satisfaction with its use in absorbable nasal packs.”
One RCT of patients (n = 205) undergoing tympanoplasty for adhesive otitis media reported that there were no complications
or adverse events in the group receiving MeroGel (which contains HA).
136
The authors did not specify what complications were
expected after surgery but did report that no patient suffered a sensorineural hearing loss or needed a reoperation.

Material Performance Study - Hyaluronic Acid
|
50
One RCT of patients (n = 55) undergoing bilateral endoscopic sinus surgery compared an absorbable crosslinked HA hydrogel
with a gelatin sponge control.
137
The authors reported that no adverse events related to the packing treatment occurred
during the study but did not report what adverse events may have been expected.
One RCT of patients (n = 227) undergoing surgery for unilateral primary chronic dacryocystitis (an infection of the lacrimal
sac, secondary to obstruction of the nasolacrimal duct) compared MeroGel with no MeroGel.
138
The authors did not report any
adverse events related to MeroGel, but did not report what adverse events may have been expected.
Systemic Responses
No systemic responses were reported in the included studies.
Overall Quality of Evidence
The SRs and RCTs were consistent in observing that there are no adverse events or complications related to HA-containing
products. The study authors noted that HA reduces local inflammatory responses and reduces scarring after surgery. The
quality of the evidence is moderate for local responses since the majority of studies are RCTs and is very low for systemic
responses (no studies investigated systemic effects).
Barrier gel for oral lesions
Three human studies (one SR, two RCTs), no animal studies. For more information, see Table 4 in Appendix D.
Local Responses/Device Events (human studies)
One SR examined HA as an adjunct treatment for chronic inflammatory disease.
99
The SR included 25 controlled studies with
studies examining HA’s effect on gingivitis, chronic periodontitis, dental surgery, and oral ulcers. No adverse events occurred
with HA use. The authors noted that HA “suppresses the immune response preventing excessive exacerbations of
inflammation.” HA’s effect on inflammation may be responsible for faster wound healing and reduction in postoperative
discomfort.
One RCT using a split-mouth design (n = 24) examined HA’s effect on moderate to severe chronic periodontitis.
139
No adverse
events occurred in the HA group. Plaque index, gingival index, papillary bleeding index, and periodontal probing depth
improved in HA-treated and control areas but was significantly better in HA-treated areas.
One RCT using a split-mouth design (n = 28) examined HA’s effect on plaque-induced gingivitis.
140
The authors’ reported “no
adverse effects to the gel were observed on clinical examination and as reported by the patients.” Plaque index, gingival
index, and gingival bleeding were examined after treatment. Inflammation was reduced with all treatments, but the reduction
was greater in HA-treated areas.
Systemic Responses
No systemic responses were reported in the included studies.
Overall Quality of Evidence
The SRs and RCTs were consistent in not observing any adverse events or complications related to HA containing products.
The quality of the evidence is moderate for local response since all studies were RCTs or controlled studies and were
consistent and is very low for systemic responses (no studies investigated).
Bulking agents
Three human studies (three RCTs), no animal studies. For more information, see Table 5 in Appendix D.
Local Host Responses (human studies)
One RCT of pediatric patients (n = 60) with grades III and IV primary vesicoureteral reflux used dextranomer HA to treat the
condition as opposed to surgery.
141
Patients were followed for 1 year. Late local complications included 6 (20%) urinary tract
infections in dextranomer HA patients and 5 (16.7%) in surgery patients.
One RCT of patients with fecal incontinence (n = 206) compared dextranomer HA with a sham injection procedure.
142
Patients
were followed for 1 year. In this study proctalgia (14%, pain due to a spasm of the pelvic floor muscles, the muscles of the

Material Performance Study - Hyaluronic Acid
|
51
anal sphincter, or the muscles of the rectum), rectal hemorrhage (7%), and diarrhea (5%) were seen in the treatment group
but were not common in the sham control group (3% proctalgia, 1% rectal hemorrhage, and 4% diarrhea).
One RCT of women (n = 344) with urinary incontinence (reported in a SR) examined mid-urethral dextranomer HA (Zuidex)
injections compared with collagen injections.
143
Patients were followed for 1 year. Dextranomer HA treated patients had higher
rates of injection site complications (16% versus 0%). Injection site sterile abscess (8.4%), injection site mass (4.4%) and
pseudo-cyst formation (2.2%) were only seen in Zuidex-treated women. Zuidex has been withdrawn from the market.
Systemic Responses
One RCT of pediatric patients (n = 60) with grades III and IV primary vesicoureteral reflux used dextranomer HA to treat the
condition as opposed to surgery.
141
Fever was noted in several patients within 2 weeks of the procedure (3 dextranomer HA
patients [10%] and 6 surgery patients [20%]).
One RCT of patients with fecal incontinence (n = 206) compared dextranomer HA with a sham injection procedure.
142
Patients
were followed for 1 year. In this study, fever (8%) was seen in the treatment group but was not seen in the sham control
group.
Overall Quality of Evidence
The quality of the evidence is moderate for local response and for systemic responses since all studies were RCTs and results
were mostly consistent.
Intravesical agents for bladder pain syndrome/interstitial cystitis therapy
Two human studies (1 SR, 1 RCT), no animal studies. For more information, see Table 6 in Appendix D.
Local Host Responses (human studies)
One SR assessed HA and HA plus chondroitin sulphate for reducing the occurrence of recurrent bacterial cystitis.
144
The SR
included 4 comparison studies (2 RCTs) with 143 patients. Bladder instillation of HA was well tolerated and reduced urinary
tract infections.
One RCT of patients with bladder pain syndrome (n = 72) compared intravesical treatment with HA, chondroitin sulfate, and a
combination of both and found there were no serious adverse events or patient withdrawals during the 24-month study in any
patient.
145
The authors reported specifically looking for recurrent febrile urinary tract infections, symptomatic cardiac
arrhythmias, significant nephrotoxicity, and hepatotoxicity that would require discontinuing treatment.
Systemic Responses
No systemic responses were reported in the included studies.
Overall Quality of Evidence
The quality of the evidence is low for local response since only 2 of the 4 studies in the SR were RCTs and the evidence base
is small and is very low for systemic responses (no studies investigated).
Organ spacer
Two human studies (no RCTs, two case series), no animal studies. For more information, see Table 7 in Appendix D.
Local Host Responses (human studies)
One case series of patients (n = 60) undergoing high-dose-rate interstitial brachytherapy for prostate cancer reported that no
adverse effects occurred after an HA injection into the perirectal fat prior to brachytherapy.
146
The authors did not report
which adverse effects they may have expected.
One case series of patients (n = 36) undergoing hypofractionated radiation therapy for prostate cancer reported that
injections of HA were well tolerated with one patient each experiencing persistent pain at the injection site, lower abdomen
pain and hematuria (blood in the urine).
147

Material Performance Study - Hyaluronic Acid
|
52
Systemic Responses
One case series of patients (n = 36) undergoing hypofractionated radiation therapy for prostate cancer reported that
injections of HA were well tolerated with one patient experiencing asthenia (abnormal physical weakness or lack of energy).
147
Overall Quality of Evidence
The quality of the evidence is low for both local responses and systemic responses because the evidence base has only two
small studies. Studies of this size are not sufficient to allow a full spectrum of adverse events to be seen.
Protective topical agent for esophageal and gastric lesions in GERD
One human study (one RCT), no animal studies. For more information, see Table 8 in Appendix D.
Local Host Responses (human studies)
One double-blind, placebo-controlled RCT of patients (n = 154) with non-erosive reflux disease treated with proton pump
inhibitors reported no serious adverse events related to using an HA plus chondroitin sulphate formulation to coat the
esophagus after a meal.
148
Mild gastrointestinal and respiratory (cough, rhinitis, throat irritation) adverse events were
somewhat more common in HA-treated patients.
Systemic Responses
The same RCT reported some mild systemic adverse events occurring only in HA-treated patients: 3 nervous system, 1
vertigo, and 1 hypertension.
Overall Quality of Evidence
The quality of the evidence is low for both local responses and systemic responses because the evidence base has only one
study but it was of medium size.
Vocal cord/fold medialization
One human study (1 SR), no animal studies. For more information, see Table 9 in Appendix D.
Local Host Responses
One SR with 14 case series (n = 442) assessed injection laryngoplasty with commercial preparations of HA to treat unilateral
vocal fold paralysis.
149
The authors noted that HA was safe for this procedure and that one patient experienced hematoma
and one patient experienced edema of the aryteno-epiglottic fold and false vocal fold. Some included studies reported local
hypersensitivity and inflammation that the authors believe may have been reactions to proteins produced in the HA
manufacturing process. Outcomes and adverse events will likely vary depending on injection technique and brand of product
used.
Overall Quality of Evidence
The quality of the evidence is low for local responses and very low for systemic responses (no studies investigated). The SR
included only case series and reported on only 442 patients.
ECRI Surveillance Data
There were no relevant reports found in ECRI’s PSO, accident investigation, or PRN databases related to devices composed of
SM-PFAS. Healthcare Technology Alerts search returned 4 manufacturer issued alerts describing problems including
compromised sterility and off label use.
Refer to Appendix F for a list of devices that guided our searches of ECRI Surveillance Data.
Patient Safety Organization
Search Results: ECRI PSO identified 11 reports that involved adhesion barrier composed of HA that occurred between
December 2016 and August 2021; however, none were pertinent to the topic of this report.
Material Performance Study - Hyaluronic Acid
|
53
Accident Investigations
Search Results: Zero investigations were recovered from the accident investigations database.
ECRI Problem Reports
Search Results: The search returned zero reports submitted by ECRI members.
Healthcare Technology Alerts
Search Results: The search returned 4 manufacturer issued alerts describing problems with sterility, labeling, and off-label
use, summarized in Table 13.
Table 13: Summary of Regulatory and Manufacturer Alerts - Adhesion Barrier and Bulking Agent Applications
Device Type
# Alerts
Reported Problem
MCN (Barrier,
Absorbable, Adhesion)
2 manufacturer issued
• Defective packaging compromises sterility.
• Recall; off-label use may lead to serious complications.
KHJ (Polymer, ENT
Synthetic-Polyamide
[Mesh or Foil Material])
1 manufacturer issued
• Mislabeled as sterile, when only syringe contents are sterile.
Adhesion Barrier
1 manufacturer issued
• Cannulae may not meet required sterility level.
Potential Gaps
ECRI surveillance searches reflect mostly acute patient incidents that involved medical devices made of Hyaluronic Acid. Areas
of particular concern involve incidents that result in direct tissue exposure to the material if there is moderate to high-quality
evidence of acute or systemic reaction to this exposure, as determined by the systematic review. Topics with very low or low
quality of evidence represent areas of potential gaps in the literature. If the literature revealed areas of new concern (e.g.,
systemic response to long-duration contact) and there is little supporting evidence, these are considered gaps.
For 7 of 9 device categories, no local adverse events were statistically significantly more likely in patients receiving HA devices
compared to patients receiving non-HA devices. This is predominantly because of a lack of studies with strong design that
investigated these events. The exceptions were adhesion barrier and bulking agents.
For 7 of 9 device categories, no systemic adverse events were statistically significantly more likely in patients receiving HA
device compared to patients received non-HA devices. This is predominantly because of a lack of studies with strong design
that investigated these events. The exceptions were adhesion barrier and bulking agents.
Only one study related to HA as a material was identified. However, because treated patients received HA as well as
chondroitin sulfate, one cannot determine whether the reported adverse events were due to HA or chondroitin sulfate or other
factors
For 8 of 9 device categories, only study involving adhesion barriers investigated the cellular or molecular mechanisms for
systemic manifestations.

Material Performance Study - Hyaluronic Acid
|
54
Appendix A. Inclusion/Exclusion Criteria and Quality of
Evidence Criteria
Inclusion Criteria
1. English language publication
2. Published between January 2011 and August 2021
3. Human studies (animal studies that provide unique information will also be considered for inclusion)
4. Systematic reviews, randomized controlled trials, cohort studies, case-control studies, cross-sectional studies, case
series
5. Studies that evaluate toxicity/biocompatibility of HA or priority devices that include this material
Exclusion Criteria
1. Foreign language publication
2. Published before January 2011
3. Not a study design of interest (e.g., in vitro lab study, case report, narrative review, letter, editorial)
4. Off-topic study
5. On-topic study that does not address a key question
6. No device or material of interest
7. No relevant outcomes (adverse events or biocompatibility not reported)
8. Study is superseded by more recent or more comprehensive systematic review
Quality of Evidence Criteria
1. Quality of comparison – is there evidence from systematic reviews including randomized and/or matched study
data and/or randomized or matched individual studies?
2. Quantity of data – number of systematic reviews and individual studies providing relevant data, as well as the
proportion of included studies that reported a specific outcome.
3. Consistency of data – are the findings consistent across studies that report relevant data?
4. Magnitude of effect – what is the likelihood of adverse effects compared to controls (with no device, lower dosage,
shorter exposure time), and possibly number of patients likely to have harms.
5. Directness of evidence – do human studies isolate the effect of the device (i.e. can the adverse effects be
attributed to the device)?
6. Is there evidence of a dose response or time response (e.g. adverse effects increase with longer exposure time)?
Material Performance Study - Hyaluronic Acid
|
55
Appendix B1. Search Summary – Muscle/Skeletal Applications
Strategies crafted by ECRI’s medical librarians combine controlled vocabulary terms and free-text words in conceptual search
statements that are joined with Boolean logic (AND, OR, NOT).
Most medical bibliographic databases such as Medline and Embase include detailed controlled vocabularies for medical
concepts accessible through an online thesaurus. Controlled vocabularies are a means of categorizing and standardizing
information. Many are rich ontologies and greatly facilitate information transmission and retrieval. Frequently seen examples
of controlled vocabularies include ICD-10, SNOMED-CT, RxNorm, LOINC, and CPT/HCPCS.
Citations in PubMed are indexed with MeSH terms and those in Embase are indexed with terms from EMTREE. These terms
are assigned either by a medical indexer or an automated algorithm. Several terms are selected to represent the major
concept of the article – these are called “major” headings. This “major” concept can be included in search strategies to limit
search retrieval. The syntax in Embase for this is /mj. We have used this convention in our strategies sparingly since indexing
is subjective and we are using a sensitive search approach which errs in the direction of comprehensiveness.
Database providers build functionality into their search engines to maximize the usefulness of indexing. One of the most
frequently used shortcuts is term explosion. “Exploding” in the context of hierarchical controlled vocabularies means typing in
the broadest (root or parent) term and having all the related more specific terms included in the search strategy with a
Boolean OR relationship. We use term explosions whenever feasible for efficiency. Feasibility depends on whether you wish to
include all of the related specific terms in your strategy. For example, in one of our approaches we explode the Emtree
concept mechanics. This explosion automatically added the all the following terms (n=174) and their associated entry terms
(lexical variants and synonyms) to the strategy using an “OR” without the searcher having to type them in. That’s one of the
major advantages to searching using controlled vocabularies. We don’t rely exclusively on controlled vocabulary terms since
there are possible limitations such as inconsistent indexing and the presence of unindexed content. That’s why we also include
free text words in our strategies.
Material: Hyaluronic Acid (HA) - Muscle/Skeletal Applications
Set
Number
Concept Search Statement
Material
1.
Hyaluronic acid (HA) and
derivatives
'hyaluronic acid'/de OR 'hyaluronic acid derivative'/de OR hyaluronan?:ti,ab
OR hyaluronate?:ti,ab OR hyaluronic:ti,ab
2.
HA viscosupplement devices
('adant' OR 'suprahyal' OR 'agili-c' OR 'artflex' OR 'arthrum' OR
'chondrotissue' OR 'cingal' OR 'crespine gel' OR 'curavisc' OR 'durolane' OR
'euflexxa' OR 'nuflexxa' OR 'gel-one' OR 'gel-syn' OR 'gelsyn' OR 'gel-syn-3'
OR 'gelsyn-3' OR 'sinovial' OR 'genvisc 850' OR 'happycross' OR 'hyalgan' OR
'hyalofast' OR 'hyalograft-c' OR 'hyalone' OR 'hyalubrix' OR 'hyalonect' OR
'hyaluron hexal' OR 'hymovis' OR 'hyadd' OR 'monovisc' OR 'neovisc' OR
'orthovisc' OR 'ostenil' OR 'ostenil tendon' OR 'recosyn' OR 'recosyn forte' OR
'recosyn uno' OR 'recosyn md' OR 'sportvis' OR 'rheovital' OR 'rheo vital' OR
'spinevisc' OR 'supartz' OR 'supartz fx' OR 'synocrom' OR 'synocrom mini' OR
'synocrom forte' OR 'synocrom forte one' OR 'structovial' OR 'synojoynt' OR
'hyalrheuma' OR 'synvisc' OR 'synvisc-one' OR 'hylan g-f 20' OR 'triluron' OR
'hyalgen' OR 'trivisc' OR 'visco-3' OR 'viscoseal'):ti,ab,kw,de,tn,dn
3.
HA bone filler devices
('aft dbm' OR 'biosphere flex' OR '45s5 bg' OR '45s5 bioglass' OR 'bioglass
45s5' OR 'confirm dbm' OR 'confirm bioactive' OR (dbx AND bone) OR 'dbx
inject' OR 'dbx strip' OR 'sygnal dbm' OR 'hyaloss' OR 'inqu paste mix' OR
'kinex' OR 'mtf new bone void filler' OR 'scs 17-01' OR
'tactoset'):ti,ab,kw,de,tn,dn
Material Performance Study - Hyaluronic Acid
|
56
4.
HA other devices
('agili-c' OR 'biopoly' OR 'chondrotissue' OR 'hyalograft*'):ti,ab,kw,de,tn,dn
5.
HA + general device and
musculoskeletal terms
#1 AND ('viscosupplementation'/exp OR 'intraarticular drug
administration'/exp OR 'musculoskeletal system inflammation'/exp OR
'musculoskeletal injury'/exp/mj OR 'bone matrix'/exp OR 'joint'/exp/mj OR
joint*:ti OR ((intra NEXT/1 articul*):ti) OR intraarticul*:ti OR osteoarthr*:ti
OR 'arthritis':ti)
6.
Combine and Limit by
language and publication
date
(#2 OR #3 OR #4 OR #5) AND [english]/lim AND [2011-2021]/py
7.
Limit by publication type
#6 NOT ('book'/it OR 'chapter'/it OR 'conference abstract'/it OR
'conference paper'/it OR 'conference review'/it OR 'editorial'/it OR
'erratum'/it OR 'letter'/it OR 'note'/it OR 'short survey'/it OR 'tombstone'/it)
Material Response
8.
'biocompatibility'/de OR biocompat* OR tribolog* OR 'bio compat*'
OR 'biological* compat*' OR 'biological* evaluation'
9.
'degradation'/exp OR degrad* OR adsorbable OR split* OR wear OR
deteriorat* OR atroph* OR migrat* OR distend* OR distension OR
'delamination'/exp OR delamina* OR leach* OR filter* OR seep* OR
evaginat* OR subsidence
10.
Leachable* OR extractable*
11.
(swell* OR shrink* OR contract* OR stretch* OR retract* OR extension OR
extend* OR deform* OR creep OR plasticity OR degrad* OR disintegrat* OR
fail* OR fragment* OR debond*) NEAR/3 (hydrogel* OR implant* OR
prosthes* OR prosthetic* OR injectable* OR putty OR putties OR graft OR
device?)
12.
'mechanics'/exp
13.
'device material'/exp/mj
14.
'Biomedical and dental materials'/exp/mj
15.
Combine sets
#8 OR #9 OR #10 #11 OR #12 OR #13 OR #14
16.
HA + Material Response
#7 AND #15
Host Response
17.
Host NEAR/2 (reaction* OR response*)
18.
‘toxicity’/exp OR toxic*:ti OR cytotox* OR teratogenic* OR genotox* OR
‘carcinogenicity’/exp OR carcinogen*:ti
Material Performance Study - Hyaluronic Acid
|
57
19.
'immune response'/exp OR 'immunity'/exp/mj OR 'hypersensitivity'/exp OR
'immunopathology'/exp/mj
20.
(immun*:ti OR autoimmun*:ti OR hypersens*:ti) NOT immunofluorescenc*:ti
21.
'inflammation'/exp OR (inflamm* NEAR/3 (tissue OR macrophage* OR
cytokine* OR react* OR respons* OR level* OR sign* OR effect* OR activat*
OR local OR inhibit* OR alleviat* OR reduc* OR decreas* OR induce* OR
synovial))
22.
'foreign body' OR granuloma* OR 'foreign body'/exp OR 'macrophage'/exp
OR 'macrophage*':ti,ab OR fouling OR 'anti-fouling' OR biofilm?
23.
'adhesion'/exp OR 'tissue adhesion'/exp OR 'tissue response' OR 'tissue
reaction' OR 'necrosis':de OR 'necrosis':ti,ab
24.
protrude* OR protrus* OR perforat*
25.
'fibrosis'/exp OR 'seroma'/exp OR 'hematoma'/exp OR 'seroma*' OR
'hematoma*' OR 'thrombosis'/exp OR 'thrombosis'/syn OR 'phlebitis'/exp OR
'phlebitis'/syn OR 'skin irritation'/exp OR 'pruritus'/exp OR 'pruritus' OR
itch*:ti,ab
26.
Combine sets
#17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25
Other combinations
27.
Combine sets
HA + Material
Response+ Host
Response
#16 AND #26
28.
HA general devices +
Host response
#5 AND #7 AND #26
29.
Combine sets
#27 OR #28
30.
HA systematic reviews
#7 AND ('systematic review'/de OR 'meta analysis'/de OR ((meta NEAR/2
analy*):ti) OR 'systematic review':ti)
31.
Final set
#29 OR #30
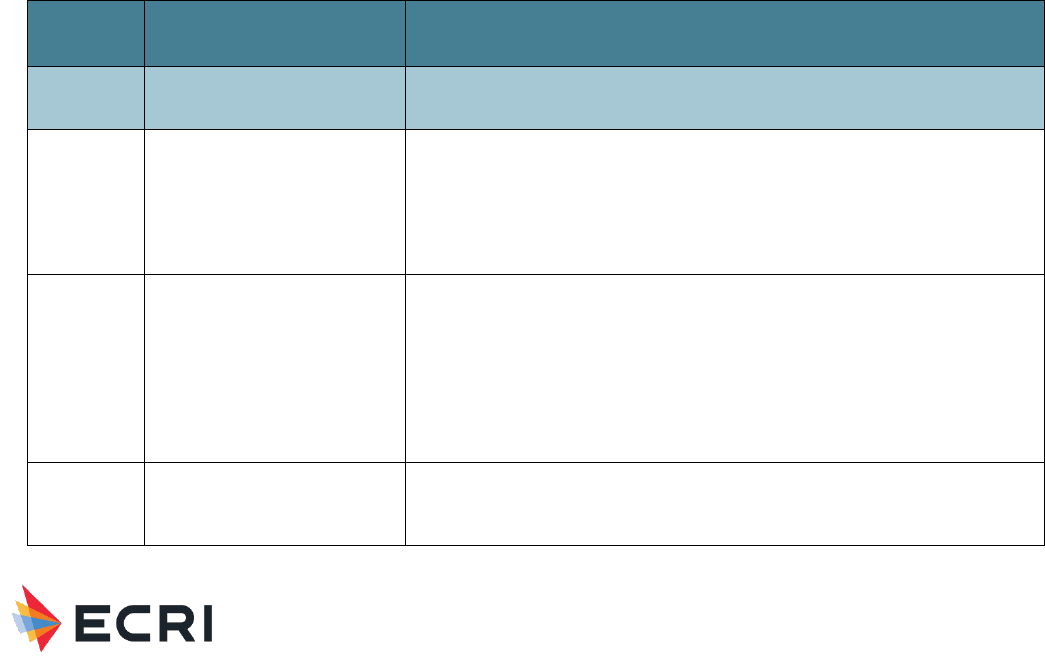
Material Performance Study - Hyaluronic Acid
|
58
Appendix B2. Search Summary – Dermal, Facial and Eye
Applications
Strategies crafted by ECRI’s medical librarians combine controlled vocabulary terms and free-text words in conceptual search
statements that are joined with Boolean logic (AND, OR, NOT).
Most medical bibliographic databases such as Medline and Embase include detailed controlled vocabularies for medical
concepts accessible through an online thesaurus. Controlled vocabularies are a means of categorizing and standardizing
information. Many are rich ontologies and greatly facilitate information transmission and retrieval. Frequently seen examples
of controlled vocabularies include ICD-10, SNOMED-CT, RxNorm, LOINC, and CPT/HCPCS.
Citations in PubMed are indexed with MeSH terms and those in Embase are indexed with terms from EMTREE. These terms
are assigned either by a medical indexer or an automated algorithm. Several terms are selected to represent the major
concept of the article – these are called “major” headings. This “major” concept can be included in search strategies to limit
search retrieval. The syntax in Embase for this is /mj. We have used this convention in our strategies sparingly since indexing
is subjective and we are using a sensitive search approach which errs in the direction of comprehensiveness.
Database providers build functionality into their search engines to maximize the usefulness of indexing. One of the most
frequently used shortcuts is term explosion. “Exploding” in the context of hierarchical controlled vocabularies means typing in
the broadest (root or parent) term and having all the related more specific terms included in the search strategy with a
Boolean OR relationship. We use term explosions whenever feasible for efficiency. Feasibility depends on whether you wish to
include all of the related specific terms in your strategy. For example, in one of our approaches we explode the Emtree
concept mechanics. This explosion automatically added the all the following terms (n = 174) and their associated entry terms
(lexical variants and synonyms) to the strategy using an “OR” without the searcher having to type them in. That’s one of the
major advantages to searching using controlled vocabularies. We don’t rely exclusively on controlled vocabulary terms since
there are possible limitations such as inconsistent indexing and the presence of unindexed content. That’s why we also include
free text words in our strategies.
Material: Hyaluronic Acid (HA) – Dermal, Facial and Eye Applications
Set
Number
Concept Search Statement
1.
Hyaluronic acid (HA) and
derivatives
'hyaluronic acid'/de OR 'hyaluronic acid derivative'/de OR hyaluronan?:ti,ab OR
hyaluronate?:ti,ab OR hyaluronic:ti,ab
2.
HA ophthalmic viscosurgical
devices (OVDs)
(clearvisc OR healon OR healon5 OR endocoat OR vitrax OR staarvisc OR optivisc
OR anikavisc OR nuvisc OR viscoat OR duovisc OR discovisc OR provisc OR
amvisc OR biolon OR eyefill* OR 'eye fill' OR visthesia OR vcombivisc OR
twinvisc OR 'z-hyalcoat' OR 'z-hyalin' OR 'z-hyalon' OR ophthalin OR 'bio-hyalur'
OR biohyalur OR microvisc OR ivisc OR optheis OR rayvisc OR shellgan OR
opelead OR opegan):ti,ab,kw,de,tn,dn
3.
HA dermal filler devices
(juvederm* OR restylane* OR perlane OR revanesse OR hydrelle OR
elevess OR prevelle OR captique OR hylaform OR 'hylan b' OR teosyal OR
'rha 2' OR 'rha 3' OR 'rha 4' OR 'rha2' OR 'rha3' OR 'rha4' OR 'belotero' OR
macrolane OR bagovit OR hyalorepair OR hyalsense OR ovita OR 'ovita fine'
OR 'ovita hv' OR dermalax OR biohyalux OR elravie OR 'art filler' OR esthelis
OR mesolis OR fortelis OR modelis OR hyaluderm OR succeev OR 'z-fill' OR
zfill OR 'princess volume' OR 'saypha volume' OR profhilo OR aliaxin OR
viscoderm OR dermalive OR surgiderm OR hydrafill):ti,ab,kw,de,tn,dn
4.
HA + general device and
ocular terms
#1 AND ('eye disease'/exp OR 'vitrectomy'/exp OR 'ophthalmic viscosurgical
device'/exp OR (eye OR eyes OR eyelid* OR ocular* OR periocular* OR
opthalm* OR 'vitreous' OR vitrectom*):ti)
Material Performance Study - Hyaluronic Acid
|
59
Set
Number
Concept Search Statement
5.
HA + general device and
dermal terms
#1 AND ('face'/exp OR 'nose'/exp OR 'skin'/exp OR 'dermal implant'/exp OR
'injectable dermal implant'/exp OR 'plastic surgery implant'/exp OR (dermal
OR skin OR face OR forehead OR eyelid* OR cheek* OR chin OR lips OR
nasolabial):ti)
6.
Combine sets
#2 OR #3 OR #4 OR #5
7.
Limit by language and
publication date
#6 AND [english]/lim AND [2011–2021]/py
8.
Limit by publication type
#7 NOT ('book'/it OR 'chapter'/it OR 'conference abstract'/it OR 'conference
paper'/it OR 'conference review'/it OR 'editorial'/it OR 'erratum'/it OR
'letter'/it OR 'note'/it OR 'short survey'/it OR 'tombstone'/it)
Material Response
9.
'biocompatibility'/de OR biocompat* OR tribolog* OR 'bio compat*'
OR 'biological* compat*' OR 'biological* evaluation'
10.
'degradation'/exp OR degrad* OR adsorbable OR split* OR wear OR
deteriorat* OR atroph* OR migrat* OR distend* OR distension OR
'delamination'/exp OR delamina* OR leach* OR filter* OR seep* OR
evaginat* OR subsidence
11.
Leachable* OR extractable*
12.
(swell* OR shrink* OR contract* OR stretch* OR retract* OR extension OR
extend* OR deform* OR creep OR plasticity OR degrad* OR disintegrat* OR
fail* OR fragment* OR debond*) NEAR/3 (hydrogel* OR implant* OR
prosthes* OR prosthetic* OR injectable* OR putty OR putties OR graft OR
device?)
13
‘mechanics’/exp
[see Emtree explosions section at the end of the strategy]
14.
‘device material’/exp/mj
15.
‘Biomedical and dental materials’/exp/mj
16.
Combine sets
#9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15
Host Response
17.
Host NEAR/2 (reaction* OR response*)
18.
‘toxicity’/exp OR toxic*:ti OR cytotox* OR teratogenic* OR genotox*
‘carcinogenicity’/exp OR carcinogen*:ti
19.
‘immune response’/exp OR ‘immunity’/exp/mj OR ‘hypersensitivity’/exp OR
‘immunopathology’/exp/mj
Material Performance Study - Hyaluronic Acid
|
60
20.
(immun*:ti OR autoimmun*:ti OR hypersens*:ti) NOT immunofluorescenc*:ti
21.
'inflammation'/exp OR (inflamm* NEAR/3 (tissue OR macrophage* OR cytokine*
OR react* OR respons* OR level* OR sign* OR effect* OR activat* OR local OR
inhibit* OR alleviat* OR reduc* OR decreas* OR induce* OR synovial))
22.
'foreign body' OR granuloma* OR 'foreign body'/exp OR 'macrophage'/exp OR
'macrophage*':ti,ab OR fouling OR 'anti-fouling' OR biofilm?
23.
'adhesion'/exp OR 'tissue adhesion'/exp OR 'tissue response' OR 'tissue
reaction' OR 'necrosis':de OR 'necrosis':ti,ab
24.
protrude* OR protrus* OR perforat*
25.
'fibrosis'/exp OR 'seroma'/exp OR 'hematoma'/exp OR 'seroma*' OR
'hematoma*' OR 'thrombosis'/exp OR 'thrombosis'/syn OR 'phlebitis'/exp OR
'phlebitis'/syn OR 'skin irritation'/exp OR 'pruritus'/exp OR 'pruritus' OR
itch*:ti,ab
26.
Combine sets
#17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR
#25
Other Combinations
27
HA + Material
Response + Host
Response
#8 AND #15 AND #26
28
HA general devices +
Host response
(#4 OR #5) AND #8 AND #26
29
Combine sets
#27 OR #28
30.
HA systematic reviews
#8 AND ('systematic review'/de OR 'meta analysis'/de OR ((meta NEAR/2
analy*):ti) OR 'systematic review':ti)
31
Combine all
#29 OR #30
Material Performance Study - Hyaluronic Acid
|
61
Appendix B3. Search Summary – Adhesion Barrier and Bulking
Agent Applications
Strategies crafted by ECRI’s medical librarians combine controlled vocabulary terms and free-text words in conceptual search
statements that are joined with Boolean logic (AND, OR, NOT).
Most medical bibliographic databases such as Medline and Embase include detailed controlled vocabularies for medical
concepts accessible through an online thesaurus. Controlled vocabularies are a means of categorizing and standardizing
information. Many are rich ontologies and greatly facilitate information transmission and retrieval. Frequently seen examples
of controlled vocabularies include ICD-10, SNOMED-CT, RxNorm, LOINC, and CPT/HCPCS.
Citations in PubMed are indexed with MeSH terms and those in Embase are indexed with terms from EMTREE. These terms
are assigned either by a medical indexer or an automated algorithm. Several terms are selected to represent the major
concept of the article – these are called “major” headings. This “major” concept can be included in search strategies to limit
search retrieval. The syntax in Embase for this is /mj. We have used this convention in our strategies sparingly since indexing
is subjective and we are using a sensitive search approach which errs in the direction of comprehensiveness.
Database providers build functionality into their search engines to maximize the usefulness of indexing. One of the most
frequently used shortcuts is term explosion. “Exploding” in the context of hierarchical controlled vocabularies means typing in
the broadest (root or parent) term and having all the related more specific terms included in the search strategy with a
Boolean OR relationship. We use term explosions whenever feasible for efficiency. Feasibility depends on whether you wish to
include all of the related specific terms in your strategy. For example, in one of our approaches we explode the Emtree
concept mechanics. This explosion automatically added the all the following terms (n = 174) and their associated entry terms
(lexical variants and synonyms) to the strategy using an “OR” without the searcher having to type them in. That’s one of the
major advantages to searching using controlled vocabularies. We don’t rely exclusively on controlled vocabulary terms since
there are possible limitations such as inconsistent indexing and the presence of unindexed content. That’s why we also include
free text words in our strategies.
Material: Hyaluronic Acid (HA) – Adhesion Barrier and Bulking Agent Applications
Set
Number
Concept Search Statement
1.
Hyaluronic acid (HLA) and
derivatives
'hyaluronic acid'/de OR 'hyaluronic acid derivative'/de OR hyaluronan?:ti,ab
OR hyaluronate?:ti,ab OR hyaluronic:ti,ab
2.
HLA adhesion barriers,
packing
('acp gel' OR advacoat OR aftamed OR carbylan* OR 'endo gel' OR epidisc
OR epifilm OR gelclair OR gengigel OR 'guardix sol' OR hyacorp OR
hyalobarrier OR hyfence OR hylasine OR intergel OR 'incert ' OR 'incert-s' OR
lubricoat OR merogel* OR meropack* OR pureregengel OR 'pureregen gel'
OR sepracoat* OR seprafilm OR sepragel OR sepramesh OR sepraspray OR
silsos OR versawrap* OR 'defehere' OR 'protahere' OR 'protad' OR
'singclean'):ti,ab,kw,de,tn,dn
3.
HLA vocal cord augmentation
('silkvoice' OR 'silk voice' OR 'hystem*'):ti,ab,kw,de,tn,dn
4. HLA bulking/spacer
('barrigel' OR 'deflux' OR 'solesta' OR 'zuidex' OR 'dexell' OR 'urodex' OR
'vurdex'):ti,ab,kw,de,tn,dn
5.
HLA Devices: Adhesion
barrier terms
#1 AND ('adhesion barrier'/exp OR 'adhesion barrier film'/exp OR 'adhesion
barrier gel'/exp OR 'anti-adhesion solution'/exp OR 'adhesion'/exp OR
'peritoneum adhesion'/exp OR 'tissue adhesion'/exp OR 'uterus synechia'/exp
OR 'anastomotic device'/exp OR 'surgical mesh'/exp OR 'adhes*':ti,ab OR
Material Performance Study - Hyaluronic Acid
|
62
Set
Number
Concept Search Statement
'adher*':ti,ab OR 'antiadhes*' OR 'anti-adhes*':ti,ab OR 'barrier*':ti,ab OR
sealant*:ti,ab OR 'mesh':ti,ab OR 'nasal packing':ti,ab OR 'nasal splint*':ti,ab)
6.
HLA Devices: Vocal cord
augmentation terms
#1 AND ('vocal cord paralysis'/exp OR 'vocal cord disorder'/exp OR 'vocal
cord'/exp OR 'vocal fold scarring'/exp OR 'vocal fold atrophy'/exp OR
'dysphonia'/exp OR (vocal NEAR/3 (fold* OR cord* OR inject* OR augment*
OR laryngoplasty OR medialization OR bulk*)) OR 'injection laryngoplasty' OR
'medialization laryngoplasty')
7.
HLA Devices: Bulking agent
terms
#1 AND ('anti vesicoureteral reflux gel'/exp OR 'feces incontinence
device'/exp OR 'injectable incontinence implant'/exp OR 'feces
incontinence'/exp OR 'urine incontinence'/exp OR 'hydrogel organ
spacer'/exp OR 'prostate immobilization device'/exp OR 'spacer balloon'/exp
OR (bulking NEAR/3 (agent* OR material* OR substance* OR inject* OR
procedure* OR treatment* OR therapy)) OR 'vesico-ureteral reflux' OR
'vesicoureteral reflux' OR 'anti-vesico-ureteral reflux' OR 'anti-vesicoureteral
reflux' OR (spacer OR spacers):ti,ab)
8.
Combine sets
#2 OR #3 OR #4 OR #5 OR #6 OR #7
9.
Limit by language and
publication date
#8 AND [english]/lim AND [2011–2021]/py
10.
Limit by publication type
#9 NOT ('book'/it OR 'chapter'/it OR 'conference abstract'/it OR 'conference
paper'/it OR 'conference review'/it OR 'editorial'/it OR 'erratum'/it OR
'letter'/it OR 'note'/it OR 'short survey'/it OR 'tombstone'/it)
Material Response
11.
'biocompatibility'/de OR biocompat* OR tribolog* OR 'bio compat*'
OR 'biological* compat*' OR 'biological* evaluation'
12.
'degradation'/exp OR degrad* OR adsorbable OR split* OR wear OR
deteriorat* OR atroph* OR migrat* OR distend* OR distension OR
'delamination'/exp OR delamina* OR leach* OR filter* OR seep* OR
evaginat* OR subsidence
13
Leachable* OR extractable*
14.
(swell* OR shrink* OR contract* OR stretch* OR retract* OR extension OR
extend* OR deform* OR creep OR plasticity OR degrad* OR disintegrat* OR
fail* OR fragment* OR debond*) NEAR/3 (hydrogel* OR implant* OR
prosthes* OR prosthetic* OR injectable* OR packing OR splint? OR device?)
15.
‘mechanics’/exp
[see Emtree explosions section at the end of the strategy]
16.
‘device material’/exp/mj
17.
‘Biomedical and dental materials’/exp/mj
18.
Combine sets
#11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17
Material Performance Study - Hyaluronic Acid
|
63
Host Response
19.
Host NEAR/2 (reaction* OR response*)
20.
‘toxicity’/exp OR toxic*:ti OR cytotox* OR teratogenic* OR genotox*
‘carcinogenicity’/exp OR carcinogen*:ti
21.
‘immune response’/exp OR ‘immunity’/exp/mj OR ‘hypersensitivity’/exp OR
‘immunopathology’/exp/mj
22.
(immun*:ti OR autoimmun*:ti OR hypersens*:ti) NOT immunofluorescenc*:ti
23.
'inflammation'/exp OR (inflamm* NEAR/3 (tissue OR macrophage* OR
cytokine* OR react* OR respons* OR level* OR sign* OR effect* OR activat*
OR local OR inhibit* OR alleviat* OR reduc* OR decreas* OR induce* OR
synovial))
24.
'foreign body' OR granuloma* OR 'foreign body'/exp OR 'macrophage'/exp
OR 'macrophage*':ti,ab OR fouling OR 'anti-fouling' OR biofilm?
25.
'adhesion'/exp OR 'tissue adhesion'/exp OR 'tissue response' OR 'tissue
reaction' OR 'necrosis':de OR 'necrosis':ti,ab
26.
protrude* OR protrus* OR perforat*
27.
'fibrosis'/exp OR 'seroma'/exp OR 'hematoma'/exp OR 'seroma*' OR
'hematoma*' OR 'thrombosis'/exp OR 'thrombosis'/syn OR 'phlebitis'/exp OR
'phlebitis'/syn OR 'skin irritation'/exp OR 'pruritus'/exp OR 'pruritus' OR
itch*:ti,ab
28.
Combine sets
#19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR
#27
Other Combinations
29
HLA + Material
Response + Host
Response
#10 AND #18 AND #28
30
HLA general devices +
Host response
(#6 OR #7) AND #10 AND #28
31
Combine sets
#29 OR #30
32.
HLA systematic reviews
#10 AND ('systematic review'/de OR 'meta analysis'/de OR ((meta NEAR/2
analy*):ti) OR 'systematic review':ti)
33
Combine all
#31 OR #32

Material Performance Study - Hyaluronic Acid
|
64
Example Embase Explosion
Mechanics/exp
• Biomechanics
• Compliance (physical)
o Bladder compliance
o Blood vessel compliance
Artery compliance
Vein compliance
o Heart muscle compliance
Heart left ventricle compliance
Heart ventricle compliance
o Lung compliance
• Compressive strength
• Dynamics
o Compression
o Computational fluid dynamics
o Decompression
Explosive decompression
Rapid decompression
Slow decompression
o Gravity
Gravitational stress
Microgravity
Weight
• Body weight
o Birth weight
High birth weight
Low birth weight
• Small for date infant
• Very low birth weight
o Extremely low birth weight
• Body weight change
o Body weight fluctuation
o Body weight gain
Gestational weight gain
o Body weight loss
Emaciation
o Body weight control
o Fetus weight
o Ideal body weight
o Lean body weight
o Live weight gain
• Dry weight
• Fresh weight
• Molecular weight
• Organ weight
o Brain weight
o Ear weight
o Heart weight

Material Performance Study - Hyaluronic Acid
|
65
o Liver weight
o Lung weight
o Placenta weight
o Spleen weight
o Testis weight
o Thyroid weight
o Uterus weight
• Seed weight
• Tablet weight
• Thrombus weight
Weightlessness
o Hydrodynamics
Hypertonic solution
Hypotonic solution
Isotonic solution
Osmolality
• Hyperosmolality
• Hypoosmolality
• Plasma osmolality
• Serum osmolality
• Urine osmolality
Osmolarity
• Blood osmolarity
• Hyperosmolarity
• Hypoosmolarity
• Plasma osmolarity
• Serum osmolarity
• Tear osmolarity
• Urine osmolarity
Osmosis
• Electroosmotic
• Osmotic stress
o Hyperosmotic stress
o Hypoosmotic stress
o Photodynamics
Photoactivation
• Photoreactivation
Photodegradation
Photoreactivity
• Photocytotoxicity
• Photosensitivity
• Photosensitization
• Phototaxis
• Phototoxicity
Photostimulation
o Proton motive force
o Shock wave
High-energy shock wave
o Stress strain relationship
o Thermodynamics
Adiabaticity
Enthalpy
Entropy

Material Performance Study - Hyaluronic Acid
|
66
• Elasticity
o Viscoelasticity
o Young modulus
• Force
• Friction
o Orthodontic friction
• Hardness
• Kinetics
o Adsorption kinetics
o Flow kinetics
Electroosmotic flow
Flow rate
Gas flow
Laminar airflow
Laminar flow
Powder flow
• Angle of repose
• Hausner ration
Pulsatile flow
Shear flow
Thixotropy
Tube flow
Turbulent flow
Vortex motion
Water flow
o Motion
Coriolis phenomenon
Rotation
Vibration
• Hand arm vibration
• High frequency oscillation
• Oscillation
• Oscillatory potential
• Whole body vibration
o Velocity
Acceleration
Deceleration
Processing speed
Wind speed
• Mass
o Biomass
Fungal biomass
Immobilized biomass
Microbial biomass
o Body mass
o Bone mass
o Dry mass
o Fat free mass
o Fat mass
o Heart left ventricle mass
o Kidney mass
• Materials testing
• Mechanical stress

Material Performance Study - Hyaluronic Acid
|
67
o Contact stress
o Contraction stress
o Shear stress
o Surface stress
o Wall stress
• Mechanical torsion
• Molecular mechanics
• Plasticity
• Pliability
• Quantum mechanics
o Quantum theory
• Rigidity
• Torque
• Viscosity
o Blood viscosity
Plasma viscosity
o Gelatinization
o Shear rate
o Shear strength
o Shear mass
o Sputum viscosity
• Viscoelasticity

Material Performance Study - Hyaluronic Acid
|
68
Appendix C1. Study Flow Diagram – Muscle/Skeletal
Applications
I. 1,795 Citations were identified by searches, of which:
1. 991 citations were not screened manually due to likely irrelevance (based on text mining, logistic
regression, etc.)
2. The remaining 804 citations were screened for potential inclusion at title/abstract level (529 citations
were selected by text mining in Distiller (30%); and 275 additional citations were selected - 89 by logistic
regression (5%) and 186 for including “random” or “systematic” in the title or abstract)
a. 377 citations were excluded at the title/abstract level. Citations excluded at this level were off-
topic, or not published in English, or did not address a Key Question, or did not report a device of
interest, or did not report an outcome of interest.
b. The remaining 427 full length citations were reviewed, of which:
i. 189 citations were excluded at 1st pass full article level, Citations excluded at this level
were off-topic, or not published in English, or did not address a Key Question, or did not
report a device of interest, or did not report an outcome of interest, or were not
available.
ii. The remaining 238 citations were reviewed, of which:
1. 199 citations were excluded at the prioritization level. Citations excluded at
this level were animal, single-arm or nonrandomized comparative studies; or were
individual studies already represented in a systematic review; or were systematic reviews
superseded by a more comprehensive systematic review.
2. 39 citations were included.

Material Performance Study - Hyaluronic Acid
|
69
Appendix C2. Study Flow Diagram – Dermal, Facial and Eye
Applications
1,401 Citations were identified by searches, of which:
1. 862 citations were not screened manually due to likely irrelevance (based on text mining, logistic regression, etc.)
2. The remaining 539 citations were screened for potential inclusion at title/abstract level (420 citations were
selected by text mining in Distiller (30%); and 119 additional citations were selected – 70 by logistic regression (5%)
and 49 for including “random” or “systematic” in the title or abstract)
a. 297 citations were excluded at the title/abstract level. Citations excluded at this level were off-topic, or
not published in English, or did not address a Key Question, or did not report a device of interest, or did not
report an outcome of interest.
b. The remaining 242 full length citations were reviewed, of which:
i. 80 citations were excluded at 1st pass full article level, Citations excluded at this level were off-
topic, or not published in English, or did not address a Key Question, or did not report a device of
interest, or did not report an outcome of interest, or were not available.
ii. The remaining 162 citations were reviewed, of which:
1. 104 citations were excluded at the prioritization level. Citations excluded at this level
were animal, single-arm or nonrandomized comparative studies; or were individual studies
already represented in a systematic review; or were systematic reviews superseded by a
more comprehensive systematic review.
2. 58 citations were included.
Appendix C3. Study Flow Diagram – Adhesion Barrier and
Bulking Agent Applications
1,217 Citations were identified by searches, of which:
1. 718 citations were not screened manually due to likely irrelevance (based on text mining, logistic regression, etc.)
2. The remaining 499 citations were screened for potential inclusion at title/abstract level (365 citations were
selected by text mining in Distiller (30%); and 134 additional citations were selected – 61 by logistic regression (5%)
and 73 for including “random” or “systematic” in the title or abstract)
a. 167 citations were excluded at the title/abstract level. Citations excluded at this level were off-topic, or
not published in English, or did not address a Key Question, or did not report a device of interest, or did not
report an outcome of interest.
b. The remaining 332 full length citations were reviewed, of which:
i. 77 citations were excluded at 1st pass full article level, Citations excluded at this level were off-
topic, or not published in English, or did not address a Key Question, or did not report a device of
interest, or did not report an outcome of interest, or were not available.
ii. The remaining 255 citations were reviewed, of which:

Material Performance Study - Hyaluronic Acid
|
70
1. 204 citations were excluded at the prioritization level. Citations excluded at this level
were animal, single-arm or nonrandomized comparative studies; or were individual studies
already represented in a systematic review; or were systematic reviews superseded by a
more comprehensive systematic review.
2. 52 citations were included.
Material Performance Study - Hyaluronic Acid
|
71
Appendix D1. Evidence Tables – Muscle/Skeletal Applications
Table 14: Viscosupplementation – knee: Health Effect (In Vivo) Human Studies
Local Response/ Toxicity
Hyaluronic acid (HA) injections
Source Citation: Carico et al. 2021
2
Study Design: SR - MAUDE
Device or Material: HA injection
Contact Duration: NR
Dose: NR
Frequency/Duration: NR
Response: Pain after injection, Purulent aspirate, Swelling
Patient characteristics (gender, mean age): NR
Number per group: 63 total unique fatalities. Knee viscosupplementation when reported.
Observed adverse effects: 8 fatalities possibly related to HA injection: Patient developed pain after injection and a
purulent aspirate before dying. Patient had swelling that led to incision of the knee and subsequent death.
Patient had pain and swelling after injection, died for unreported reasons after admission. Suicide following
worsening pain.
Timing of adverse effects: Timing NR for fatalities associated with local adverse events.
Factors that predict response: Patients with risk factors for infections, such as immunocompromised patients since
most fatalities were often associated with infectious processes vs. the effects of the HA.
Source Citation: Sedrak et al. 2021
3
Study Design: SR; Case Report
Device or Material: HA for IA injection (89% receiving Synvisc [HA] Sanofi-Aventis)
Contact Duration: SR: up to 6 months Case report: 4 weeks
Dose: NR
Frequency/Duration: SR: 2 to 4+ administrations; Case report: single administration
Response: Pseudoseptic arthritis presenting with: Effusion Pain, acute, Synovial leucocyte elevation
Patient characteristics (gender, mean age): 85.2% female, 60.8 years (31-79).
Number per group: SR (11 studies: 5 single arm studies, 6 case reports), 28 knees (27 patients) with acute
pseudoseptic arthritis after HA injection for knee OA.
Case report: 1 knee (1 patient).
Observed adverse effects: SR: All patients presented with acute pain and joint effusion. 24 of 28 cases underwent
arthrocentesis, with 3 cases (12.5%) having synovial leucocyte elevation in the range typically concerning
Material Performance Study - Hyaluronic Acid
|
72
for septic arthritis, and another 16 cases (66.7%) with elevations in an inflammatory cell count range of
5,000 to 50,000. Case report: progressive and increasing pain with obvious suprapatellar effusion.
Timing of adverse effects: SR: 22 cases (78.6%) of pseudoseptic arthritis presented within 24 hours of injection; 15
of the 22 cases (68.2%) presented within the first 12 hours. Time from injection to presentation ranged
from 1 hour to 9 days. 7 cases (25%) occurred after 2
nd
injection, 5 cases (17.9%) occurred after 3
rd
injection, and 13 cases (46.4%) occurred after fourth or greater injection. 16 of 17 cases (57.1%) reported
significant improvement in clinical and laboratory tests within 3 weeks. 2 cases (7.1%) did not mention
improvement by 6 months. Case report: symptoms at 12 hours post-injection with significant improvement
in acute flare of pain and swelling by week 1. By week 4, complete resolution of acute inflammatory
symptoms.
Factors that predict response: NR
Source Citation: Park et al. 2020
18
Study Design: RCT
Device or Material: Hyaluronic acid injections (HA; not specified) vs. PRP injections
Contact Duration: 6 months
Dose: NR
Frequency/Duration: Single administration
Response: Musculoskeletal pain, Swelling and tenderness of knee joint
Patient characteristics (gender, mean age): 78% female; 62.3 years HA, 60.6 years PRP.
Number per group: 55 each arm, mild-to-moderate knee OA.
Observed adverse effects: No serious AEs were reported, and no significant differences were reported for mild-to-
moderate AEs. Musculoskeletal pain (3 HA, 1 PRP), and mild tenderness and mild swelling of knee joint
occurred in both arms – see below for timing and N.
Timing of adverse effects: Mild swelling: At 6 weeks: HA: 28 mild, 27 moderate (100%); PRP: 30 mild, 25 moderate
(100%)). At 3 months (HA: 28 mild, 19 moderate (85%); PRP: 29 mild, 24 moderate (96%)). At 6 months
(HA: 30 mild, 18 moderate (87%); PRP: 28 mild, 22 moderate (91%)). Mild tenderness: Baseline and 6
weeks: 2 HA (baseline and 6 weeks) and 1 PRP (baseline).
Factors that predict response: NR
Source Citation: Raeissadat et al. 2020
19
Study Design: RCT
Device or Material: Hyalgan® injections (FidiaFarmaceutici S.p.A, Abano Terme, Italy) vs. PRP-derived growth factor
(PRGF) injections
Contact Duration: 12 months
Dose: NR
Frequency/Duration: 3 weekly injections of Hyalgan, 2 PRGF injections 3 weeks apart
Response: Heaviness of injection site, Stiffness
Patient characteristics (gender, mean age): 58.63±7.09 HA, 57.08±7.3 PRGF, 71% female.

Material Performance Study - Hyaluronic Acid
|
73
Number per group: 119 patients (59 HA, 60 PRGF) with mild-to-moderate knee OA (Kellgren and Lawrence grade 2
to 3); at 12 months 52 HA, 50 PRGF.
Observed adverse effects: Minor complications including swelling (0 HA), stiffness, and heaviness of injection site
occurred in 3 (5.9%) Hyalgan, 10 (19.6%) PRGF
Timing of adverse effects: NR
Factors that predict response: NR
Source Citation: Di et al. 2018
4
Study Design: Systematic review
Device or Material: HA injections (unspecified) vs. PRP (4 frozen, 3 fresh; 4 leukocyte-poor PRP, 3 leukocyte-rich
PRP)
Contact Duration: mean 9 months (6 months to 12 months)
Dose: NR
Frequency/Duration: HA (NR), PRP (2 to 4 injections)
Response: Post-injection pain, Swelling
Patient characteristics (gender, mean age): 56% females, 59.8 years
Number per group: 7 RCTs, 908 patients with mild knee OA. Sample size 55 to 183; mean 128.
Observed adverse effects: 2 studies reported no AEs. 1 study reported significantly more post injection pain with PRP
(p=0.039). 1 study reported no significant difference in AEs with 92% of AEs in the HA arm not related to
treatment. 1 study reported no significant difference in AEs. 16 AEs (8 per arm) were all related to pain
associated with infiltration. 1 study reported severe pain and swelling with HA, but significantly more post-
injection swelling and pain overall with PRP. 1 study reported no significant differences in infiltration-related
AEs which were infrequent, mild and appeared immediately after injection.
Timing of adverse effects: AEs immediately after injection.
Factors that predict response: NR
Source Citation: Li et al. 2018
5
Study Design: Systematic review
Device or Material: Intra-articular HA (IAHA; unspecified) vs intra-articular oxygen-ozone (manufacturer NR)
Contact Duration: mean 9 months (range 6 to 12 months)
Dose: HA dose: 10 mg/ml x 2.0 ml (3 studies), 10 mg/ml x 2.5 ml (1 study); Ozone dose: 30 ug/ml (3 studies), 35
ug/ml (1 study)
Frequency/Duration: NR
Response: Bleeding, Local skin reaction
Patient characteristics (gender, mean age): 75% female, 60 to 71 years.
Number per group: 4 RCTs (sample size 42 to 141). 298 (151 HA, 147 oxygen-ozone) patients with end-staged knee
OA.
Observed adverse effects: Included local skin reaction and bleeding. No significant difference in incidence rate of AEs
(risk difference 0.006, 95% CI: -0.047 to 0.058; p=0.837).

Material Performance Study - Hyaluronic Acid
|
74
Timing of adverse effects: NR
Factors that predict response: NR
Source Citation: Altman et al. 2016
6
Study Design: Systematic review
Device or Material: IAHA injections of various molecular weights (HMW ≥3000 kDa, MMW <3000 and >1500 kDa,
LMW ≤1500 kDa); biological fermentation derived HA (Bio-HA) or avian-derived HA (AD-HA); included
Euflexxa, Synvisc, Supartz, Orthovisc, and Durolane
Contact Duration: NR
Dose: NR
Frequency/Duration: NR
Response: Effusion after injection, Flare ups
Patient characteristics (gender, mean age): NR
Number per group: 30 RCTs. 11 RCTs (n=2094) addressed HMW. 4 RCTs (n=621) addressed MMW. 15 RCTs
(n=2639) addressed LMW. 1776 patients received Bio-HA treatment, while 3070 received AD-HA treatment.
Observed adverse effects: Significantly lower incidence of acute flare ups at the injection site with AD-HA (54
(3.04%) Bio-HA vs. 405 (13.19%); p<0.05). HMW products had the highest rate of injection site flare-ups.
Significantly more injection flare ups with HMW vs. MMW (13.73% vs. 3.31%; p<0.001) and HMW vs. LMW
(13.73% vs. 10.73%; p<0.001). Significantly more flare ups with LMW vs. MMW (10.73% vs. 3.31%;
p=0.007). No significant difference in effusion after injection based on molecular weights (1.9% HMW,
1.7% MMW, 1.8% LMW), but significantly higher incidence of effusion with AD-HA (3.44% AD-HA, 0.54%
Bio-HA; p<0.001).
Timing of adverse effects: NR
Factors that predict response: NR
Source Citation: Zhao et al. 2016
7
Study Design: Systematic review
Device or Material: Hylan G-F 20 (Synvisc, relatively higher molecular weight) vs. LMWHAs (Artzal, Orthovisc, Bio-HA,
Hyalgan, Sinovial, Variofil, Hydros)
Contact Duration: mean 26.8 weeks (range 3 to 54 weeks)
Dose: average molecular weight (kd): range 500 to 3600
Frequency/Duration: 1 injection (2 RCTs), 2 injections (1 RCT), and 3 injections (17 RCTs)
Response: Treatment-related adverse events
Patient characteristics (gender, mean age): gender NR, mean 62.8 years.
Number per group: 10 RCTs (n=2616, 2711 knees) were included in the safety analysis. Synvisc: n=1,203 (1,249
knees), LMWHA: n=1,413 (1,462 knees). Knee OA.
Observed adverse effects: No significant difference in patients with TRAEs (14.7% Synvisc vs. 11.6% LMWHA; risk
difference 0.02, 95% CI: -0.01 to 0.05; p=0.13) or number of TRAEs (23.2% Synvisc vs. 16.3% LMWHA;
risk difference 0.03, 95% CI: -0.01 to 0.07; p=0.14).
Timing of adverse effects: NR
Material Performance Study - Hyaluronic Acid
|
75
Factors that predict response: NR
Source Citation: Miller et al. 2020
8
Study Design: Systematic review
Device or Material: IAHA (Synvisc, Hyalgan, Suplasyn, Suvenyl, Durolane) vs. oral NSAIDs (naproxen, diclofenac,
loxoprofen, etoricoxib)
Contact Duration: mean 17.8 weeks (range 5 to 26 weeks)
Dose: NR
Frequency/Duration: HA: 1 injection (1 study of Durolane), 3 weekly (3 studies of Synvisc, and Suplasyn), and 5
weekly (2 studies of Hyalgan and Suvenyl); NSAIDS: naproxen 500 mg bid, diclofenac 100 mg qd and 75
mg bid, loxoprofen 60 mg tid, etoricoxib 60 mg qd
Response: Arthralgia, Effusion, Injection site pain
Patient characteristics (gender, mean age): 36% to 72% females; 57 to 69 years.
Number per group: 6 RCTs (sample size 60 to 327 patients). 831 patients with mild-to-moderate knee OA; 414 HA,
417 NSAIDs.
Observed adverse effects: Significantly lower risk of AEs with HA (5 studies, 19.8% vs. 29%; risk ratio 0.74, 95% CI:
0.59 to 0.94; p=0.01. Significantly higher risk of arthralgia (8.1% vs. 2.9%; p=0.001) with HA. No
significant difference in risk of serious AE (5 studies, 1.2% vs. 0.9%, RR 1.37, 95% CI: 0.26 to 7.14;
p=0.71). Lower leg effusion rarely occurred (1 (0.3%) HA, 2 (0.5%) NSAIDs).
Timing of adverse effects: NR
Factors that predict response: NR
Source Citation: He et al. 2017
10
Study Design: Systematic review
Device or Material: IAHA (Hyalgan, Orthovisc, Synvisc, Ostenil, NASHA, Hylastan SGL-80, HYADD 4, sodium
hyaluronate, HA) vs. intraarticular corticosteroids (CS; triamcinolone hexacetonide, methylprednisolone
acetate, 6-methylprednisolone acetate, triamcinolone acetonide)
Contact Duration: mean 4.8 months (range 1 to 6 months)
Dose: 16 mg (Synvisc, HA), 20 mg (Hyalgan, Ostenil), 25 mg (sodium hyaluronate, 30 mg (Orthovisc), 60 mg
(NASHA)
Frequency/Duration: Single injection of NASHA , Synvisc, and HA; 1 or 2 weekly injections of Hyalston; 2 weekly
injections of HYADD 4; 3 weekly injections of Orthovisc and Synvisc; 5 weekly injections of Hyalgan, sodium
hyaluronate, and Ostenil
Response: Joint stiffness, Joint swelling, Knee pain
Patient characteristics (gender, mean age): 62% females, range 49 to 71 years.
Number per group: 12 RCTs (n=1794), sample size: 41 to 391 patients with knee OA. 971 HA, 823 CS; 6 RCTs
(n=1352) reported TRAEs
Observed adverse effects: Significantly more TRAEs with HA (746 events vs. 606 events; risk ratio 1.66, 95% CI:
1.34 to 2.06; p<0.00001) which may have been caused by higher injection frequency of HA.
Timing of adverse effects: NR

Material Performance Study - Hyaluronic Acid
|
76
Factors that predict response: NR
Source Citation: Bannuru et al. 2014
11
Study Design: Systematic review
Device or Material: IAHA injections including Synvisc (Genzyme, Ridgefield, NJ), Hyalgan (Fidia Pharmaceutical
Corporation, Abano Terme, Italy), Suplaysn (Bioniche Life Sciences Inc., Belleville, Ontario), and Suvenyl
(Chugai Pharmaceutical Corp., Tokyo, Japan) vs. NSAIDs including diclofenac, naproxen, loxoprofen
Contact Duration: mean 10.4 weeks (range 4 to 12 weeks)
Dose: HA (NR)
Frequency/Duration: 3 or 5 weekly injections pf IAHA
Response: None reported
Patient characteristics (gender, mean age): females range 36% to 68%, range 61 to 67 years.
Number per group: 5 RCTs (n=712), sample size 51 to 325. Knee OA. 357 HA, 355 NSAID.
Observed adverse effects: 3 SAEs (2 myocardial infarctions, 1 transient ischemic attack) but unrelated to IAHA. 1
SAE (related GI bleed) with NSAIDs
Timing of adverse effects: N/A
Factors that predict response: NR
Source Citation: Ke et al. 2021
20
Study Design: RCT
Device or Material: Hylan G-F 20 injections (Synvisc, Sanofi) vs. saline injections
Contact Duration: 26 weeks
Dose: 6 ml, 48 mg hylan polymer
Frequency/Duration: Single injection
Response: Arthralgia, Edema, peripheral, Injection-site edema, joint pain, pain, swelling, Joint swelling, peripheral
Patient characteristics (gender, mean age): 78% female, 61.5 years (All East Asian ethnicity)
Number per group: 440 (220 each arm). Mostly mild-to-moderate knee OA.
Observed adverse effects: No difference in treatment-emergent adverse events (TEAEs (61.5% Synvisc, 64.5%
placebo). AEs later considered treatment-related were mostly musculoskeletal and connective tissue
disorders (e.g., arthralgia, peripheral joint swelling). TEAEs more commonly reported with Synvisc included
injection site pain (1 (0.5%) vs. 0), joint swelling (8 (3.7%) vs. 2 (0.9%), injection site edema (1 (0.5%) vs.
0), injection site swelling (1 (0.5%) vs. 0), and edema peripheral (1 (0.5%) vs 0). Arthralgia (7 (3.2%) each
arm) and injection site joint pain (1 (0.5%) occurred similarly. Injection site joint swelling only occurred with
placebo (1 (0.5%)). No serious TRAEs were reported.
Timing of adverse effects: NR
Factors that predict response: NR
Source Citation: Miller et al. 2019
12
Material Performance Study - Hyaluronic Acid
|
77
Study Design: Systematic review
Device or Material: IAHA (Hyalgan, Durolane, Euflexxa, Hya-Ject, Orthovisc, Artzal, Monovisc, Kartilage Cross, Go-On,
Suplasyn, NRD101, Gel-One) vs. intraarticular saline injections
Contact Duration: median 6 months (range 5 weeks to 2 years)
Dose: molecular weight (kd) ≥6000 (7 studies), <1500 to <6000 (6 studies), ≤1500 (9 studies)
Frequency/Duration: injections: 1 (7 studies), 3 (13 studies), 4 (3 studies), 5 (11 studies), 11 (1 study)
Response: Arthralgia, Injection site pain, Joint effusion, Joint swelling
Patient characteristics (gender, mean age): female range 22 to 100% overall, age range 53 to 72 overall
Number per group: 35 RCTs (n=8078), sample size <100 per arm (12 studies), ≥100 per arm (13 studies) 4295
IAHA, 3783 saline.
Observed adverse effects: No difference in risk of AEs (42.4% vs. 39.7%; Risk ratio 1.01, 95% CI: 0.96 to 1.07;
p=0.61), SAEs (1.8% vs. 1.2%; RR 1.44, 95% CI: 0.91 to 2.26, p=0.12). Significantly more local non-
serious AEs with IAHA (14.5% vs. 11.7%; RR 1.21, 95% CI: 1.07 to 1.36; p=0.003). Most common local
reactions were injection site pain, arthralgia, joint swelling, and joint effusion which mostly subsided within
2 to 3 days.
Timing of adverse effects: Up to 3 days post-injection for injection site pain, arthralgia, joint swelling, and joint
effusion.
Factors that predict response:NR
Source Citation: Honvo et al. 2019
13
Study Design: Systematic review
Device or Material: Injections of hyaluronic acid, sodium hyaluronate, and Hyalgan vs. intraarticular saline injections
Contact Duration: 25 to 29 weeks (8 RCTs), 52 weeks (1 RCT)
Dose: 20 mg, 25 mg, 60 mg, 1 ml, 2 ml, 30 mg/2 ml
Frequency/Duration: 1 cycle; number of injections per cycle was 1 (1 study), 3 (3 studies), 5 (4 studies), and 11 (1
study)
Response: Treatment-related serious AEs, Treatment-related severe AEs
Patient characteristics (gender, mean age): gender NR, range 62 to 66 years.
Number per group: 9 RCTs (n=1967), 6 RCTs on knee OA, 3 RCTs on ankle OA; for safety analysis. 991 IAHA, 976
saline/placebo.
Observed adverse effects: All TRAEs Significantly increased odds of treatment-related serious AEs with IAHA (80 HA,
42 placebo; OR 1.78, 95% CI: 1.21 to 2.63), but no significant differences in any severe AEs (25 HA, 26
placebo; OR 1.08, 95% CI: 0.50 to 2.31; p=0.270).
Timing of adverse effects: NR
Factors that predict response: NR
Source Citation: Petterson and Plancher 2019
21
Study Design: RCT
Device or Material: Lightly cross-linked IAHA (Monovisc™) vs. intraarticular saline injections

Material Performance Study - Hyaluronic Acid
|
78
Contact Duration: 2 to 26 weeks
Dose: 88 mg
Frequency/Duration: Single administration
Response: Arthralgia, Joint stiffness, Joint swelling
Patient characteristics (gender, mean age): 58% female, 59 years.
Number per group: 369 patients (184 Monovisc, 185 saline) with mild-to-moderate knee OA (Kellgren Lawrence
grade II and III).
Observed adverse effects: No significant difference in incidence of arthralgia (7 (3.8%) in each arm), joint swelling (2
(1.1%) Monovisc, 1 (0.5%) saline), joint stiffness (1 (0.5%) Monovisc, 2 (1.1%) saline). Device-related AEs
occurred in 13 (7.1%) patients with Monovisc, and 10 (5.4%) patients with saline. 24 events (not specified)
occurred with Monovisc, and 14 events with placebo.
Timing of adverse effects: NR
Factors that predict response: NR
Source Citation: Hangody et al. 2018
22
Study Design: RCT
Device or Material: Monovisc (Anika Therapeutics) vs. saline injections
Contact Duration: 26 weeks
Dose: 4 mL, 88 mg HA
Frequency/Duration: Single administration
Response: Arthralgia
Patient characteristics (gender, mean age): 66% female with Monovisc, 74% female with saline; 59.2 years
Monovisc, 58.0 saline.
Number per group: 219 (150 Monovisc, 69 saline) patients with Kellgren-Lawrence grade I to III knee OA.
Observed adverse effects: Device-related AEs included arthralgia (2 Monovisc). No serious AEs occurred with
Monovisc. 2 SAEs occurred with saline.
Timing of adverse effects: NR
Factors that predict response: NR
Source Citation: Concoff et al. 2017
14
Study Design: Systematic review
Device or Material: IAHA of various molecular weights (including Durolane, Euflexxa, Orthovisc, Synvisc, Hyalgan,
Artzal, Gel-ONE, BioHY, NRD101 HA, Fermathron plus) vs. intraarticular saline injections
Contact Duration: mean 26 weeks (range 4 to 52 weeks)
Dose: 10 mg/2 ml to 30 mg/3 ml
Frequency/Duration: Single injections (4 RCTs, n=1196), 2 to 4 injections (16 RCTs, n=2865), ≥5 injections (11
RCTs, n=1847); 1 study administering 1-11 injections was included in 2 subgroups (2-4, and ≥5)
Response: Treatment-related adverse events (unspecified)

Material Performance Study - Hyaluronic Acid
|
79
Patient characteristics (gender, mean age): NR
Number per group: 30 RCTs (n=5848).
Observed adverse effects: Significantly more TRAEs with IAHA in studies using ≥5 injections (risk ratio (RR) 1.70,
95% CI: 1.12 to 2.59; p=0.01). No significant difference in TRAEs in studies using single injection IA-HA
(RR 1.11, 95% CI: 0.93 to 1.32; p= 0.26), or 2 to 4 injections (RR 0.98, 95% CI: 0.87 to 1.09; p=0.67). No
significant difference in number of patients experiencing a TRAE (RR 1.13, 95% CI: 0.95 to 1.35; p=0.16).
Timing of adverse effects: NR
Factors that predict response: NR
Source Citation: O'Hanlon et al. 2016
15
Study Design: Systematic review
Device or Material: HA injections (Hyalgan, Adant, Orthovisc, Synvisc, hyaluronic acid, Suplasyn, NRD101, Bio-Hy) vs.
placebo injections
Contact Duration: 26 weeks
Dose: 20 mg/2 ml; 2 mL, 15 mg/mL; 2 ml, 20 mg/ml; 0.2 mg/2 ml; 2.5 ml 1% hyaluronan; 2 ml 0.8%; single 6 ml;
16 mg/2ml
Frequency/Duration: 1-10 total treatments, time between treatments: 1 day, 1 to 3 weeks
Response: Arthralgia, Cutaneous vasculitis, Effusion, Knee pain, Knee swelling, Knee tenderness
Patient characteristics (gender, mean age): % female range 46 to 80, age range 39 to 92 years.
Number per group: All Kellgren Lawrence grades included. 8 studies (1056 HA, 1017 placebo) included in meta-
analysis on all serious AEs. 10 studies (1645 HA, 1564 placebo) included in meta-analysis on all non-serious
AEs.
Observed adverse effects: No significant difference in serious AEs (27 events HA, 16 events placebo; relative risk
1.39, 95% CI: 0.78 to 2.47) or non-serious AEs (415 events HA, 375 events placebo; RR 1.03, 95% CI: 0.93
to 1.15). 1 non-serious local reaction attributed to HA was cutaneous vasculitis in 1 patient. Other non-
serious AEs were arthralgia, effusion, transient increase in pain, swelling, and tenderness in treated knee.
Timing of adverse effects: Cutaneous vasculitis during 1
st
week post HA injection. Responses immediately post-
injection were arthralgia, effusion, transient increase in pain, swelling, and tenderness in treated knee.
Factors that predict response: NR
Source Citation: Petrella et al. 2011
23
Study Design: RCT
Device or Material: HA injections of DMW (combined HA different molecular weights and concentrations), HMW, LMW
vs. saline injections
Contact Duration: 104 weeks
Dose: DMW: 0.7 ml of sterile 2.2% LMW (0.58-0.78 x 10
6
Daltons) sodium hyaluronate and 0.7 ml of sterile 1%
HMW (1.2-2.0 x 10
6
Daltons) sodium hyaluronate. LMW: sodium hyaluronate of 500-730 kDa, 20 mg/2 mL.
HMW: sodium hyaluronate of 6000 kDa, 16 mg/2 mL
Frequency/Duration: Weekly for 3 weeks
Response: Erythema at injection site, Local swelling, Pain, Stiffness in index knee

Material Performance Study - Hyaluronic Acid
|
80
Patient characteristics (gender, mean age): 47% female, range 68 to 71 years.
Number per group: 200 (50 DMW, 50 HMW, 50 LMW, 50 saline) with K-L grade I to III.
Observed adverse effects: Non-serious AEs associated with the injection procedure included pain and local swelling at
injection site (21%), erythema at injection site (12%), and stiffness in the index knee (7%). There were no
serious AEs.
Timing of adverse effects: Immediately post-injection all HA injections. 2 reactions at 52 weeks and 1 reaction at 104
weeks with HMW.
Factors that predict response: NR
Source Citation: Chen et al. 2020
24
Study Design: RCT
Device or Material: Synvisc-One ([HA] Sanofi-Aventis) vs. cooled radiofrequency ablation (CRFA) with COOLIEF
([non-HA] Avanos Medical)
Contact Duration: 6 months
Dose: 6 mL
Frequency/Duration: Single administration
Response: Baker cyst, Pain during and after procedure
Patient characteristics (gender, mean age): Subjects presenting signs and symptoms of knee OA (84% moderate-to-
severe OA). Synvisc-One: 54% female, 63.1 years. COOLIEF: 52% female, 63.3 years.
Number per group: Synvisc-One: 88; COOLIEF: 89.
Observed adverse effects: Of 157 events (63 HA, 94 CRFA), 27 events were deemed related to treatment. In the HA
group, 9 (14%) events in 9 patients were noted as being related to treatment, versus 18 (19%) events in
13 patients in the CRFA group. Adverse-event profiles were deemed similar. Events occurring with both
treatments included: Pain during procedure: Synvisc-One 3 (3%), COOLIEF 3 (3%). Post-procedure pain:
Synvisc-One 3 (3%), COOLIEF 7 (8%). AEs only occurring with Synvisc-One: Baker’s cyst in 2 (2%)
patients, and an unspecified event in 1 patient. AEs only occurring with COOLIEF: numbness (2 (2%)),
stiffness/tightness (1 (1%)), bruising/swelling (1 (1%)), skin issue (1 (1%)).
Timing of adverse effects: Recorded at 6-month follow-up.
Factors that predict response: NR
Source Citation: Rezasoltani et al. 2020
25
Study Design: RCT
Device or Material: Hyalgan ([HA] Fidia Farmaceutici) vs. PT, dextrose prolotherapy, botulinum neurotoxin
Contact Duration: 3 months
Dose: 2 mL
Frequency/Duration: 3 injections, 1 week apart
Response: No device-related responses
Patient characteristics (gender, mean age): Overall: 62.5% females, 67.1 years (range 50 to 83 years). PT: 60%
female, 70 years. Botulinum neurotoxin: 73% female, 67.7 years. HA: 53% female, 66.1 years. Dextrose
prolotherapy: 63% female, 64.8 years.

Material Performance Study - Hyaluronic Acid
|
81
Number per group: 28 patients per group (total n = 112). At least moderate knee OA.
Observed adverse effects: After HA or toxin injection, patients remained under observation at the clinic for at least 30
minutes for possible short-term side effects including hypersensitivity and bleeding, none recorded. None of
the participants showed or reported serious side effects for the treatments.
Timing of adverse effects: NR
Factors that predict response: NR
Source Citation: Ting et al. 2020
16
Study Design: Systematic review
Device or Material: HA vs. acupotomy
Contact Duration: 3 to 5 week duration; 3 and 6 months followup
Dose: 2 mL
Frequency/Duration: Single and multiple administration
Response: Redness and swelling after injection
Patient characteristics (gender, mean age): 44% to 72% female, 40 to 78 years.
Number per group: 4 RCTs in meta-analysis. 30 to 50 each arm (total 153 HA, 156 acupotomy).
Observed adverse effects: Four RCTs reported AE incidence at 2.2% for the HA group and 4.3% in the acupotomy
group. Results for HA (2 RCTs) included no AEs, and redness and swelling post-injection in 3 patients.
Results for acupotomy (2 RCTs) included palpitation, chest tightness, and cold sweat in 4 patients; and pain,
bleeding, hematoma, and swelling in 3 patients.
Timing of adverse effects: Pain and swelling shortly after injection.
Factors that predict response: NR
Source Citation: Hermans et al. 2019
26
Study Design: RCT
Device or Material: HMW-HA (Hylan G-F 20 (Synvisc, Sanofi, S.A. Paris, France) plus usual care (UC) vs. UC
Contact Duration: Up to 52 weeks
Dose: 6000 kDa
Frequency/Duration: 3 weekly injections
Response: Knee flare up, Other AEs unspecified
Patient characteristics (gender, mean age): 50% female, 54 years.
Number per group: 156 patients with mild-to-moderate knee OA. 77 HMW-HA, 79 UC.
Observed adverse effects: 156 TRAEs occurred (77 Synvisc, 79 controls). 6 weeks: total patients experiencing a
TRAE was higher with Synvisc (40 (45%) Synvisc, 23 (18%) UC) and due to knee flare up (36% Synvisc,
10% UC), other (11% Synvisc vs. 10% UC); and were GI-related (see systemic responses below).13 weeks:
total patients experiencing a TRAE was higher with UC (15% Synvisc, 27% UC) and due to knee flare up
(8% Synvisc, 16% UC), other (8% Synvisc, 9% UC); and were GI-related (see systemic responses below).
26 weeks: total patients experiencing a TRAE was similar (16% Synvisc, 15% UC) and due to knee flare up
(8% Synvisc, 8% UC), other (5% Synvisc, 7% UC); and were GI-related (see systemic responses below). 39

Material Performance Study - Hyaluronic Acid
|
82
weeks: total patients experiencing a TRAE was similar (13% Synvisc, 11% UC) and due to knee flare up
(8% Synvisc, 7% UC), other (4% Synvisc, 1% UC); and were GI-related (see systemic responses below). 52
weeks: total patients experiencing a TRAE was lower with Synvisc (12% Synvisc, 20% UC) and due to knee
flare up (6% Synvisc, 11% UC), other (4% Synvisc, 16% UC); and were GI-related (see systemic responses
below).
Timing of adverse effects: At 6, 13, 26, 29, and 52 weeks.
Factors that predict response: NR
Source Citation: Kim et al. 2019
17
Study Design: Systematic review
Device or Material: HA vs. PDRN injections
Contact Duration: 4 to 12 months
Dose: 2 mL (8 to 30 mg/mL)
Frequency/Duration: 3 to 5 injections, 1 week apart
Response: General AEs
Patient characteristics (gender, mean age): 57.5% female. 60 to 67 years.
Number per group: 5 RCTs. Sample size 14 to 50 (total 290 patients in the meta-analysis, n = 145 each for HA and
PDRN groups). Diagnosis NR.
Observed adverse effects: The incidence of attributable AEs was low in the HA and PDRN groups, and the difference
in events between the groups was not significant (relative risk 2.15, 95% CI: 0.17 to 26.67; p=0.55).
Timing of adverse effects: NR
Factors that predict response: NR
Source Citation: Saeed et al. 2015
27
Study Design: RCT
Device or Material: IAHA injection vs. arthroscopic debridement
Contact Duration: 6 months
Dose: NR
Frequency/Duration: Weekly for 5 weeks
Response: Pain at injection site
Patient characteristics (gender, mean age): 83.3% females, range 40 to 60 years (66%), >60 years (34%).
Number per group: 120 (60 each arm) with knee OA K-L grade II and III.
Observed adverse effects: Pain at injection site in 8 (13.4%) patients with IAHA vs. pain and mild effusion in 13
(26%) patients after debridement. No serious AEs were reported.
Timing of adverse effects: Immediately post-injection/postoperative and subsided within 4 to 5 days.
Factors that predict response: NR
Systemic Response/ Toxicity
HA intra-articular injections

Material Performance Study - Hyaluronic Acid
|
83
Source Citation: Carico et al. 2021
2
Study Design: SR - MAUDE
Device or Material: HA intraarticular injection
Contact Duration: NR
Dose: NR
Frequency/Duration: NR
Response: Cellulitis, Death, Paralysis, Septic shock, Septicemia
Patient characteristics (gender, mean age): NR
Number per group: 63 total unique fatalities. Knee viscosupplementation when reported
Observed adverse effects: 8 fatalities possibly related to HA injection. Patient received HA injection, hospitalized with
septic shock, developed paralysis, and died after 4 months. Patient developed necrotizing fasciitis after
starting chemotherapy and receiving an HA injection. Patient developed septicemia, cause of death
unknown. Patient developed cellulitis following HA injection.
Timing of adverse effects: One MAUDE report detailed a patient hospitalized with septic shock who developed
paralysis and died after four months. Timing NR for other fatalities associated with systemic adverse events.
Factors that predict response: Patients with risk factors for infections, such as immunocompromised patients
Source Citation: Sedrak et al. 2021
3
Study Design: SR; Case Report
Device or Material: HA for intraarticular injection (89% receiving Synvisc [HA] Sanofi-Aventis)
Contact Duration: SR: up to 6 months Case report: 4 weeks
Dose: NR
Frequency/Duration: SR: 1 to 4+ administrations; Case report: single administration
Response: Pseudoseptic arthritis presenting with: Elevated CRP above 50, Elevated ESR above 5,000, Elevated PMN
above 75% Fever, Leukocytosis above 10,000
Patient characteristics (gender, mean age):
Number per group: 85.2% female, 60.8 years (31-79).
Observed adverse effects: SR (11 studies: 5 single arm studies, 6 case reports), 28 knees (27 patients) with acute
pseudoseptic arthritis after HA injection for knee OA. Case report: 1 knee (1 patient). SR: 6 patients
(22.2%) were reported to have fevers on presentation with other reported symptoms. 8 of 28 cases
reported the results of blood work, with a leukocytosis above 10000 noted in 4 cases (50%), and a white
blood cell count above 12,500 in one case (12.5%). Five of seven studies (71.4%) reporting CRP and ESR
reported elevated CRP above 50, and 6 of those studies (85.7%) reported having elevated ESR. 10 out of
25 cases (40%) reported leucocyte differentials with elevated PMNs above 75%.
Timing of adverse effects: SR: 22 cases (78.6%) presented within 24 hours of injection; 15 of the 22 cases (68.2%)
presented within the first 12 hours. Time from injection to presentation ranged from 1 hour to 9 days. 3
cases (10.7%) occurred after the 1
st
injection, 7 cases (25%) occurred after 2
nd
injection, 5 cases (17.9%)
occurred after 3
rd
injection, and 13 cases (46.4%) occurred after fourth or greater injection. Case report:
Arthrocentesis revealed cell count of 123,260 and WBC count of 15.5. 8 mL of serosanguinous fluid
aspirated at repeat aspiration 20 hours post-injection, with cell count of 175,960.

Material Performance Study - Hyaluronic Acid
|
84
Factors that predict response: The pathophysiological response for pseudoseptic arthritis has yet to be fully
described, though literature suggests that the accumulation of viscous material (e.g., phagocytised HA) may
contribute to the adverse event. Significantly more reactions occurred in patients receiving more than a
single administration. Time to onset of symptoms is faster than patients with septic arthritis following IA
injection (11.9 days versus 72 hours). Composition of HA injected (82.8% of cases) involved Synvisc
(including the case report) with literature suggesting these avian-derived HA preparations may have a
higher incidence of acute flare-ups and effusions in comparison to biologically fermented preparations.
Source Citation: Park et al. 2020
18
Study Design: RCT
Device or Material: HA injections (not specified) vs. PRP
Contact Duration: 6 months
Dose: NR
Frequency/Duration: Single administration
Response: Backache, Headache, Nasopharyngitis
Patient characteristics (gender, mean age): 78% female; 62.3 years HA, 60.6 years PRP
Number per group: 55 each arm, mild-to-moderate knee OA
Observed adverse effects: Nasopharyngitis occurred in both arms (2 HA, 1 PRP). 2 AEs only occurred with HA
(backache: 2 HA, 0 PRP; headache: 1 HA, 0 PRP).
Timing of adverse effects: NR
Factors that predict response: NR
Source Citation: Di et al. 2018
4
Study Design: Systematic review
Device or Material: HA injections (unspecified) vs. PRP (4 frozen, 3 fresh; 4 leukocyte-poor PRP, 3 leukocyte-rich
PRP)
Contact Duration: up to 52 weeks
Dose: NR
Frequency/Duration: HA (NR), PRP (2 to 4 injections)
Response: None reported
Patient characteristics (gender, mean age): 56% females, 59.8 years
Number per group: 7 RCTs, 908 patients with mild knee OA. Sample size 55 to 183; mean 128.
Observed adverse effects: None reported.
Timing of adverse effects: N/A
Factors that predict response: N/A
Source Citation: Miller et al. 2020
8
Study Design: Systematic review

Material Performance Study - Hyaluronic Acid
|
85
Device or Material: Intraarticular HA (Synvisc, Hyalgan, Suplasyn, Suvenyl, Durolane) vs. oral NSAIDs (naproxen,
diclofenac, loxoprofen, etoricoxib)
Contact Duration: mean 17.8 weeks (range 5 to 26 weeks)
Dose: NR
Frequency/Duration: HA: single course of 1- (1 study of Durolane), 3- (3 studies including Synvisc, Suplasyn), and 5-
(2 studies including Hyalgan and Suvenyl); NSAIDS: naproxen 500 mg bid, diclofenac 100 mg qd and 75 mg
bid, loxoprofen 60 mg tid, etoricoxib 60 mg qd
Response: Cardiac-related complications, Headache
Patient characteristics (gender, mean age): 36% to 72% females; 57 to 69 years.
Number per group: 6 RCTs (sample size 60 to 327 patients). 831 patients with mild-to-moderate knee OA; 414 HA,
417 NSAIDs,
Observed adverse effects: Significantly higher risk of headache (8.4% vs. 4.4%; p=0.03) with HA. Rarely occurring
AEs were cardiac-related complications (1 (0.3%) HA, 0 NSAID). Drug allergies/intolerance occurred in 4
(1%) NSAID.
Timing of adverse effects: NR
Factors that predict response: NR
Source Citation: Ran et al. 2018
9
Study Design: Systematic review
Device or Material: Intraarticular HA vs. intra-articular methylprednisolone (MPA)
Contact Duration: mean 30.4 weeks (range 24 to 52 weeks)
Dose: NR
Frequency/Duration: HA: 2 ml once a week for 5 weeks or 3 weeks, single injections of 3 ml or 4 ml, single injection
of 4 ml once a week for 2 weeks
Response: Headache, Nausea, Vomiting
Patient characteristics (gender, mean age): range 53% to 66% female; range 49 years to 72 years
Number per group: 5 RCTs (n=1004); sample size: 60 to 433 patients with knee OA.
Observed adverse effects: AEs in 4 RCTs included nausea, vomiting and headache. No significant difference in AE
incidence (Risk difference = -0.042, 95% CI: -0.092 to 0.009; p=0.107)
Timing of adverse effects: NR
Factors that predict response: NR
Source Citation: He et al. 2017
10
Study Design: Systematic review
Device or Material: Intraarticular HA (Hyalgan, Orthovisc, Synvisc, Ostenil, NASHA, Hylastan SGL-80, HYADD 4,
sodium hyaluronate, hyaluronic acid) vs. intraarticular corticosteroids (CS; triamcinolone hexacetonide,
methylprednisolone acetate, 6-methylprednisolone acetate, triamcinolone acetonide)
Contact Duration: mean 4.8 months (range 1 to 6 months)

Material Performance Study - Hyaluronic Acid
|
86
Dose: 16 mg (Synvisc, hyaluronic acid), 20 mg (Hyalgan, Ostenil), 25 mg (sodium hyaluronate, 30 mg (Orthovisc),
60 mg (NASHA)
Frequency/Duration: Single injection of NASHA , Synvisc, and hyaluronic acid; 1 or 2 weekly injections of Hyalston; 2
weekly injections of HYADD 4; 3 weekly injections of Orthovisc and Synvisc; 5 weekly injections of Hyalgan,
sodium hyaluronate, and Ostenil
Response: No treatment-related systemic responses
Patient characteristics (gender, mean age): 62% females, range 49 to 71 years
Number per group: 12 RCTs (n=1794), sample size range 41 to 391. 971 HA, 823 CS; 6 RCTs (n=1352) reported
treatment-related AEs.
Observed adverse effects: 3 RCTs reported serious systemic responses in either IAHA or intraarticular CS group, but
none were related to treatment or procedure.
Timing of adverse effects: NR
Factors that predict response: NR
HA vs. saline/ placebo
Source Citation: Honvo et al. 2019
13
Study Design: Systematic review
Device or Material: Injections of hyaluronic acid, or sodium hyaluronate, Hyalgan vs. placebo
Contact Duration: 25 to 29 weeks (8 RCTs), 52 weeks (1 RCT)
Dose: 20 mg, 25 mg, 60 mg, 1 ml, 2 ml, 30 mg/2 ml
Frequency/Duration: 1 cycle; number of injections per cycle was 1 (1 study), 3 (3 studies), 5 (4 studies), and 11 (1
study)
Response: System-organ class-related AEs (cardiac, GI, musculoskeletal and connective tissue, nervous system, renal
and urinary disorders; respiratory, thoracic and mediastinal; skin and subcutaneous tissue, and vascular)
Patient characteristics (gender, mean age): gender NR, range 62 to 66 years.
Number per group: 9 RCTs (n=1967), 6 RCTs on knee OA, 3 RCTs on ankle OA; for safety analysis. 991 IAHA, 976
saline/placebo.
Observed adverse effects: All treatment-related adverse events No significant differences in any system-organ
class(SOC)-related AEs (GI, cardiac, vascular, respiratory, nervous system, skin and subcutaneous tissue
disorders, musculoskeletal, renal and urinary disorders, infections and infestations, and hypersensitivity
reaction). GI disorders: 69 HA, 83 placebo; OR 0.81, 95% CI: 0.52 to 1.27; p=0.344. Cardiac disorders: 5
HA, 4 placebo; OR 1.25, 95% CI: 0.36 to 4.41; p=0.639. Vascular disorders: 5 HA, 3 placebo; OR 1.70,
95% CI: 0.39 to 7.29; p=0.650. Respiratory, thoracic and mediastinal disorders: 71 HA, 52 placebo; OR
1.21, 95% CI: 0.82 to 1.78; p=0.764. Nervous system disorders: 66 HA, 55 placebo; OR 1.15, 95% CI: 0.77
to 1.70; p=0.79. Skin and subcutaneous tissue disorders: 22 HA, 10 placebo; OR 1.71, 95% CI: 0.52 to
5.63; p=0.173. Musculoskeletal and connective tissue disorders: 145 HA, 149 placebo; OR 0.99, 95% CI:
0.71 to 1.39; p=0.225. Renal and urinary disorders: 6 HA, 12 placebo; OR 0.54, 95% CI: 0.21 to 1.41;
p=0.75. Hypersensitivity reaction: 23 HA, 19 placebo; OR 0.64, 95% CI: 0.05 to 7.94; p=0.081.
Timing of adverse effects: NR
Factors that predict response: NR
Source Citation: Hangody et al. 2018
22

Material Performance Study - Hyaluronic Acid
|
87
Study Design: RCT
Device or Material: Monovisc (Anika Therapeutics) vs. saline injections
Contact Duration: 26 weeks
Dose: 4 mL, 88 mg HA
Frequency/Duration: Single administration
Response: Rash
Patient characteristics (gender, mean age): 66% female with Monovisc, 74% female with saline; 59.2 years Synvisc,
58.0 saline.
Number per group: 219 (150 Monovisc, 69 saline) patients with Kellgren-Lawrence grade I to III knee OA.
Observed adverse effects: Device-related AEs included rash (1 Monovisc).
Timing of adverse effects: NR
Factors that predict response: NR
Source Citation: O'Hanlon et al. 2016
15
Study Design: Systematic review
Device or Material: HA injections (Hyalgan, Adant, Orthovisc, Synvisc, hyaluronic acid, Suplasyn, NRD101, Bio-Hy) vs.
Placebo
Contact Duration: 26 weeks
Dose: 20 mg/2 ml; 2 mL, 15 mg/mL; 2 ml, 20 mg/ml; 0.2 mg/2 ml; 2.5 ml 1% hyaluronan; 2 ml 0.8%; single 6 ml;
16 mg/2ml
Frequency/Duration: 1-10 total treatments, time between treatments: 1 day, 1 to 3 weeks
Response: Skin reaction
Patient characteristics (gender, mean age): % female range 46 to 80, age range 39 to 92 years
Number per group: Kellgren Lawrence all grades included. 8 studies (1056 HA, 1017 placebo) included in
metaanalysis on all serious AEs.
Observed adverse effects: 1 serious AE attributed to HA was skin reaction characterized by peeling of skin on hands
and toes occurring in 1 patient.
Timing of adverse effects: Skin reaction occurred 8 days post HA injection.
Factors that predict response: NR
HA vs. miscellaneous treatments
Source Citation: Hermans et al. 2019
26
Study Design: RCT
Device or Material: HMW-HA (Hylan G-F 20 (Synvisc, Sanofi, S.A. Paris, France) plus usual care (UC) vs. UC
Contact Duration: Up to 52 weeks
Dose: 6000 kDa
Frequency/Duration: 3 weekly injections
Response: GI complaints

Material Performance Study - Hyaluronic Acid
|
88
Patient characteristics (gender, mean age): 50% female, 54 years.
Number per group: 156 patients with mild-to-moderate knee OA. 77 HMW-HA, 79 UC.
Observed adverse effects: Systemic TRAEs included GI complaints. 6 weeks: 7% Synvisc, 6% UC, 13 weeks: 3%
Synvisc, 12% UC, 26 weeks: 5% Synvisc, 3% UC , 39 weeks: 5% Synvisc, 4% UC, 52 weeks: 4% Synvisc,
3% UC
Timing of adverse effects: At 6, 13, 26, 29, and 52 weeks.
Factors that predict response: NR
AE: adverse event; bid: twice a day; CRP: C reactive protein; ESR: erythrocyte sedimentation rate; HA: hyaluronic acid:
HMW: high molecular weight; IA: intraarticular; IAHA: intraarticular hyaluronic acid; kd: kilodalton; kDa: kilodalton; K-L:
Kellgren and Lawrence; LMW: low molecular weight; LMWHAs: low molecular weight hyaluronic acids; MAUDE: manufacturer
and user facility device experience FDA database; ml: milliliter; MMW: medium molecular weight; NR: not reported; NSAID:
nonsteroidal anti-inflammatory drug; OA: osteoarthritis; qd: 4 times a day; tid: 3 times a day; PDRN: polydeoxyribonucleotide;
PMN: polymorphonuclear leukocytes; RCT: randomized controlled trial; SR: systematic review; TRAE: treatment-related
adverse events; WBC: white blood cell.
Table 15: Viscosupplementation – hip: Health Effect (In Vivo) Human Studies
Local Response/ Toxicity
Source Citation: Liao et al. 2019
28
Study Design: Systematic review of 5 RCTs comparing HA to placebo
Device or Material: HA solution (Durolane, hylan G-F 20, Hyalubrix, Hyalgan, Adant)
Contact Duration: Final follow-up at 56 to 182 days post-procedure
Dose: HA: 2.5 ml, 3 ml, 6 ml (2 studies), 8 ml
Frequency/Duration: 1 to 3 injections
Response: Hematoma, Pain flare, Pruritus
Patient characteristics (gender, mean age): 67% female. Mean age 60 to 70. Characteristics not reported by
treatment group.
Number per group: HA (n = 298); placebo (n = 293). Note: 39% of the patients in this SR were in studies that are
also included in
31
.
Observed adverse effects: Hematoma: 1 (HA), 0 (placebo). Pain flare: 7% (HA), 2.4% (placebo). Pruritus: 1 (HA), 0
(placebo). Statistical tests of between-group differences in AEs were not performed.
Timing of adverse effects: NR
Factors that predict response: NR
Source Citation: Leite et al. 2018
29
Study Design: Systematic review of 9 RCTs comparing HA to placebo or other intra-articular treatments

Material Performance Study - Hyaluronic Acid
|
89
Device or Material: HA solution (Durolane, Hyalubrix, Synvisc-One, Hyalgan, Adant, Hylan G-F 20), corticosteroid,
PRP, mepivacaine, placebo
Contact Duration: 2 to 12 months
Dose: HA: 2 to 8 ml
Frequency/Duration: 1 to 3 injections
Response: Overall AEs
Patient characteristics (gender, mean age): Gender of patients was available for 8 of the 9 studies (508/885 = 57%
female). Mean age was given in 8 of the 9 studies (overall mean 60.9 years).
Number per group: HA (n = 558), CS (n = 201), PRP (n = 115), mepivacaine (n = 20), placebo (n = 270). Note: 1
study (n = 357) is also in Liao et al., 1 study (n = 312) is also in Wu et al., 4 studies (n = 284) are also in
both, and 3 studies (n = 223) are also in Ye et al.
Observed adverse effects: HA: 29.0% overall AE rate across 9 studies (range: 0% to 49.7%). Placebo: 23.7% rate
across 4 studies (range: 0% to 34.9%). PRP: 8.7% rate across 3 studies (range: 0% to 20%).
Corticosteroid: 38.3% rate across 3 studies (range: 0% to 48.7%). Mepivacaine: 5% in 1 study. Meta-
analysis of overall AEs was performed for comparisons of HA with placebo and methylprednisolone; no
statistically significant differences were found.
Timing of adverse effects: NR
Factors that predict response: NR
Source Citation: Ye et al. 2018
30
Study Design: Systematic review of 4 RCTs comparing HA to PRP
Device or Material: HA solution, PRP (products not specified)
Contact Duration: 12 months
Dose: HA: 1 ml – 2 ml
Frequency/Duration: NR
Response: Nausea, Post-injective pain
Patient characteristics (gender, mean age): In 3 studies reporting: HA 50.0% female; PRP 56.5% female; combined
53.4% female. Mean age: HA, 59.8; PRP, 59.0; combined, 59.4.
Number per group: HA (n = 148), PRP (n = 155).
Observed adverse effects: Nausea: RR = 1.15, 95% CI: 0.34 to 3.93. One included study reported significantly
higher post-injective pain for PRP than for HA (p = 0.043).
Timing of adverse effects: NR
Factors that predict response: NR
Source Citation: Wu et al. 2017
31
Study Design: Systematic review of 6 RCTs comparing HA to placebo and conservative treatment (Depomedrol)
Device or Material: HA solution (hylan G-F 20, Hyalubrix, Ostenil, Durolane, Adant, Hyalgan)
Contact Duration: Final follow-up at 56 to 180 days post-procedure
Dose: HA: 2 ml (2 studies), 2.5 ml, 3 ml, 6 ml, 8 ml

Material Performance Study - Hyaluronic Acid
|
90
Frequency/Duration: 1 to 3 injections
Response: Overall AEs
Patient characteristics (gender, mean age): Gender NR. Mean age 59.5-70 years. Characteristics not reported for
treatment group.
Number per group: HA (n=291); placebo (n=114); Depomedrol (n=207). Note: 44% of the patients in this SR were
in studies that are also included in Liao et al. 2019
28
.
Observed adverse effects: No significant differences between HA and “control group” (placebo and conservative
treatment combined) in overall AEs (“including transient post injection pain, superficial infection and
hematoma”); RR 0.94; 95% CI 0.41 to 2.20; p > 0.05.
Timing of adverse effects: NR
Factors that predict response: NR
AE: adverse event; CS: corticosteroid; HA: hyaluronic acid; ml: milliliter; NR: not reported; PRP: platelet-rich plasma; RCT:
randomized controlled trial; SR: systematic review
Table 16: Viscosupplementation – hand and ankle: Health Effect (In Vivo) Human Studies
Local Response/Toxicity
Source Citation: Boffa et al. 2021
32
Study Design: Systematic review of 24 studies of intra-articular injective treatments for ankle lesions. 18 studies
included HA.
Device or Material: HA solution (Euflexxa, Durolane, Suplasyn, Hanox-M-XL, Hyalgan, Synvisc, Supartz, Orthovisc,
Adant, sodium hyaluronate), PRP, corticosteroid, botulinum toxin type A, prolotherapy, mesenchymal stem
cells, placebo/saline
Contact Duration: Final follow-up at 1 to 45.5 months
Dose: HA: 1 ml to 12.5 ml
Frequency/Duration: 1 to 5 injections
Response: Enlarged lymph nodes, Pain, Pseudogout, Swelling
Patient characteristics (gender, mean age): 55% male. Mean age 35.5 to 63.9 years.
Number per group: HA (n=581), PRP (n=83), corticosteroid (n=48), botulinum toxin type A (n=38), prolotherapy
(n=27), exercise therapy (n=15), mesenchymal stem cells (n=6), saline (n=46). Of the 24 studies, 8 were
RCTs, 1 was a nonrandomized comparative study, and 15 were single-arm studies.
Observed adverse effects: One patient each in the HA group reported enlarged lymph node in ipsilateral groin,
osteochondritis dissecans (not considered treatment-related), and pseudogout. “In some cases, pain and
swelling were reported at the injection site soon after the injection.”
Timing of adverse effects: NR
Factors that predict response: NR
Source Citation: Kroon et al. 2016
33

Material Performance Study - Hyaluronic Acid
|
91
Study Design: Systematic review of 13 RCTs and “quasi-RCTs” of intra-articular therapies for hand OA. 9 studies
included HA.
Device or Material: HA solution (hylan G-F 20, sodium hyaluronate), corticosteroid, dextrose, infliximab, placebo
(saline, lidocaine, bupivacaine)
Contact Duration: 24 weeks
Dose: HA: 1 ml – 3 ml
Frequency/Duration: HA: 1-3 injections
Response: Overall AEs
Patient characteristics (gender, mean age): 87% female. Mean age 62.8 years.
Number per group: HA (n=360), corticosteroid (n=280), dextrose (n=30), infliximab (n=10), placebo (n=172). Note:
Two studies (one comparing HA with placebo, one comparing infliximab with placebo) were within-subject.
Each patient in those 2 studies (33 HA/placebo, 10 infliximab/placebo) is counted as being in both groups in
the numbers given here.
Observed adverse effects: Of the nine studies including HA, one did not report numbers of patients experiencing AEs.
In the 8 remaining studies, AE rates were as follows: HA, 11.5%; corticosteroid, 9.0%; placebo, 2.7%.
Numbers of patients with specific types of AE were not reported, either overall or by group; AEs were
described as minor, and included surgeries unrelated to study medication, pain and local swelling, skin and
nail abnormalities, heat and/or redness, and unspecified “local AEs.”
Timing of adverse effects: NR
Factors that predict response: NR
AE: adverse event; HA: hyaluronic acid; ml: milliliter; NR: not reported; OA: osteoarthritis; PRP: platelet-rich plasma: RCT:
randomized controlled trial

Material Performance Study - Hyaluronic Acid
|
92
Table 17: Viscosupplementation – shoulder: Health Effect (In Vivo) Human Studies
Local Response/ Toxicity
Source Citation: Zhang et al. 2019
34
Study Design: Systematic review of 15 studies
Device or Material: HA (Orthovisc, sodium hyaluronate, Hylan G-F 20, Euflexxa) vs anesthetic + corticosteroid, 6-
methylprednisolone, or phosphate-buffered saline. HA dose vs HA dose. 7 studies had no control.
Contact Duration: 12 weeks to 3 years
Dose: 2 mL to 8 mL
Frequency/Duration: Single injection to five weekly injections
Response: Abscess, Musculoskeletal pain, Pain at injection site
Patient characteristics (gender, mean age): Gender NR in 5 studies, range 50.5% to 76% male per arm in 10
studies; all patients older than 18 years.
Number per group: 15 studies (5 RCTs, 10 single arm), sample size for HA: range 15 to 265.
Observed adverse effects: Thirteen studies recorded adverse events after intra-articular administration of HA and
found a pooled adverse event rate of 33.92% (406 of 1197) and a serious adverse event rate of 5.35% (64
of 1197). Almost all of these events were deemed by the study investigators to not be product-related.
Common AEs from IAHA were musculoskeletal pain, and pain at injection site. Serious AEs such as severe
musculoskeletal pain and abscess were also reported. Similar findings were present in control groups
receiving intra-articular injection of corticosteroids or phosphate-buffered saline, with a reported pooled
adverse event rate of 48.88% (240 of 491) and a serious adverse event rate of 2.24% (11 of 491).
Timing of adverse effects: NR
Factors that predict response: NR
Source Citation: Lee et al. 2015
35
Study Design: Systematic review of 4 RCTs
Device or Material: HA vs intraarticular corticosteroid injection or physical therapy
Contact Duration: 2 weeks to 6 months
Dose: 20 to 30 mg
Frequency/Duration: Weekly for 2-3 weeks (2 studies), every 2 weeks for 6 weeks (1 study); 15-day intervals for the
first month, then monthly for 6 months (1 study); total injections 2 to 8
Response: Pain
Patient characteristics (gender, mean age): 69% female; 54.5 to 64.2 years.
Number per group: NR
Observed adverse effects: Rovetta and Monteforte reported no major adverse events and adverse event data could
not be obtained for 2 other included trials (Callis et al., Hsieh et al). Park et al. reported that 12 out of 45
participants undergoing capsular distension combined with intra-articular HA injection experienced pain
during the procedure, and the pain may be a result of the capsular distension procedure, the intra-articular
HA injection, or both.

Material Performance Study - Hyaluronic Acid
|
93
Timing of adverse effects: Intra-procedural.
Factors that predict response: NR
Systemic Response/ Toxicity
Source Citation: Zhang et al. 2019
34
Study Design: Systematic review of 15 studies
Device or Material: HA (Orthovisc, sodium hyaluronate, Hylan G-F 20, Euflexxa) vs anesthetic + corticosteroid, 6-
methylprednisolone, or phosphate-buffered saline. HA dose vs HA dose. 7 studies had no control.
Contact Duration: 12 weeks to 3 years
Dose: 2 mL to 8 mL
Frequency/Duration: Single injection to five weekly injections
Response: Cancer, Chest pain, Diarrhea, Flu symptoms, Headache
Patient characteristics (gender, mean age): Gender NR in 5 studies, range 50.5% to 76% male per arm in 10
studies; all patients older than 18 years.
Number per group: 15 studies (5 RCTs, 10 single arm), sample size for HA: range 15 to 265.
Observed adverse effects: Diarrhea, flu symptoms, and headache were commonly reported (data not provided).
Serious AEs such as chest pain and cancer were also reported (data not provided).
Timing of adverse effects: NR
Factors that predict response: NR
HA: hyaluronic acid; IAHA: intraarticular hyaluronic acid; ml: milliliter
Table 18: Viscosupplementation – temporomandibular joint disorders: Health Effect (In Vivo) Human Studies
Local Response/ Toxicity
Source Citation: Liu et al. 2019
36
Study Design: Systematic review
Device or Material: Hyaluronate vs. corticosteroid or placebo
Contact Duration: 1 week to 8 years
Dose: 0.5 to 1 mL
Frequency/Duration: Single administration
Response: Ear pressure, Pain after injection
Patient characteristics (gender, mean age): 50% to 88% female, 25.6 to 50.1 years.
Number per group: 14 to 102. 8 studies included.
Observed adverse effects: No relevant difference between CS and HA groups in the occurrence of adverse events. In
three studies reporting adverse events, 4 (20%), 13 (37%), and 0% of patients receiving HA injection
reported TMJ pain after injection, compared to 0%, 14 (50%), and 28 (70%) patients receiving CS injection

Material Performance Study - Hyaluronic Acid
|
94
– odds ratio 2.98, 95% CI: 0.08 to 111.17; p=0.55. Ear pressure (reported in Bjornland et al. 2007): 1 (5%)
HA; 2 (10%) CS – odds ratio 2.11, 95% CI: 0.18 to 25.35; p=0.56.
Timing of adverse effects: Ear pressure reported at 2 week follow up.
Factors that predict response: NR
Source Citation: Haigler et al. 2018
37
Study Design: Systematic review
Device or Material: HA vs. PRP or PRGF injection
Contact Duration: 12 months
Dose: 1 mL to 5mL
Frequency/Duration: Single administration; 1x/week for 3 consecutive weeks
Response: Discomfort post-op, Pain at injection site
Patient characteristics (gender, mean age): 56%-96% female; 28.1 to 38.2 years
Number per group: 13 to 50. 3 relevant RCTs.
Observed adverse effects: One of three relevant studies (an RCT) reviewed reported adverse events. Pain during
injection: 15 (60%) HA, 22 (88%) PRP or PRGF. Post-operative discomfort: 2 (8%) HA, 19 (76%) PRP or
PRGF
Timing of adverse effects: Post-injection; Immediate post-op
Factors that predict response: NR
Source Citation: Liu et al. 2019
36
Study Design: Systematic review
Device or Material: Hyaluronate vs. corticosteroid or placebo
Contact Duration: 1 week to 8 years
Dose: 0.5 to 1 mL
Frequency/Duration: Single administration
Response: Chills
Patient characteristics (gender, mean age): 50% to 88% female, 25.6 to 50.1 years.
Number per group: 14 to 102. 8 studies included.
Observed adverse effects: No relevant difference between CS and HA groups in the occurrence of adverse events.
Slight chill (reported in Shi et al. 2002): 2 (5.7%) HA; 0% CS – odds ratio 0.24, 95% CI: 0.01 to 5.90;
p=0.36. General rash (reported in Bjornland et al. 2007): 0% HA; 2 (10%) CS – odds ratio 5.54, 95% CI:
0.25 to 123.08; p=0.28.
Timing of adverse effects: 1 week (sourced in abstract of Shi et al. 2002).
Factors that predict response: NR

Material Performance Study - Hyaluronic Acid
|
95
CS: corticosteroid; HA: hyaluronic acid; mL: milliliter; NR: not reported; PRP: platelet-rich plasma; PRPG: platelet-rich growth
factor; TMJ: temporomandibular joint
Table 19: Scaffold - Health Effect (In Vivo) Human Studies
Local Response/ Toxicity
Source Citation: Glasbrenner et al. 2020
38
Study Design: RCT
Device or Material: Polyglycolic acid and hyaluronan matrix-augmented bone marrow stimulation (m-BMS;
Chondrotissue [BioTissue AG]) after microfracture (MF) vs. MF
Contact Duration: 108 weeks
Dose: NR
Frequency/Duration: Single administration
Response: Infected hematoma, Mild swelling
Patient characteristics (gender, mean age): 37.5% female, 46.0 years m-BMS, 40.5 years MF.
Number per group: 12 each arm, grade III/IV International Cartilage Repair Society (ICRS) cartilage degradation.
Observed adverse effects: Infected hematoma in 1 patient and mild swelling in 3 patients in m-BMS group. There
was only 1 severe effusion after 6 weeks in the MF group. Moderate or severe allergic reactions were not
observed in either group.
Timing of adverse effects: Infected hematoma: NR; mild swelling 2 weeks after surgery in m-BMS group, resolved
after 6 weeks.
Factors that predict response: NR
Source Citation: Sofu et al. 2019
39
Study Design: Nonrandomized comparative
Device or Material: HA-based cell-free scaffold (Hyalofast®, Anika Therapeutics Inc.) vs. chitosan-glycerol
phosphate/blood implant (BST-CarGel®, Piramal Life Sciences)
Contact Duration: Mean: 24.4±5 months
Dose: NR
Frequency/Duration: Single administration
Response: Cyst formation, Early degenerative changes, Edema, Persistent pain
Patient characteristics (gender, mean age): 52% female; 39±10 years Hyalofast, 42±11 years BST-CarGel.
Number per group: Hyalofast, n=21, BST-CarGel, n=25; focal osteochondral lesion of the knee joint (Outerbridge
grade III or IV). Both groups underwent MF.
Observed adverse effects: Edema or cyst formation in the subchondral bone was detected in 8 (38%) knees with
Hyalofast and 10 (40%) knees with BST-CarGel. One patient in both groups had persistent pain as well as
early degenerative changes of the knee joint and planned to undergo replacement surgery at the latest
follow-up. Deep venous thrombosis, septic arthritis, neurovascular complication, or intra-articular adhesion
was not detected in any patient.

Material Performance Study - Hyaluronic Acid
|
96
Timing of adverse effects: NR
Factors that predict response: NR
Source Citation: Sofu et al. 2017
40
Study Design: Nonrandomized comparative
Device or Material: HA based cell-free scaffold (Hyalofast, Hyalofast; Anika Therapeutics) + MF vs MF
Contact Duration: Mean 25.7 ± 2.5 months
Dose: NR
Frequency/Duration: Single administration
Response: Early degenerative changes, Persistent pain
Patient characteristics (gender, mean age): 62.8% female; 40 ± 9.8 years HA scaffold, 43 ± 6.8 years MF.
Number per group: HA scaffold=19, MF=24; focal osteochondral lesion of the knee joint.
Observed adverse effects: One patient from group 1 (HA scaffold) and 2 patients from group 2 (MF) had persistent
pain as well as early degenerative changes of the knee joint.
Timing of adverse effects: NR
Factors that predict response: NR
HA: hyaluronic acid; NR: not reported; RCT: randomized controlled trial

Material Performance Study - Hyaluronic Acid
|
97
Appendix D2. Evidence Tables – Dermal, Facial and Eye
Applications
Table 20: Dermal Fillers - Health Effect (In Vivo) Human Studies
Local Response/ Toxicity
Source Citation: Gorbea et al. 2021
41
Study Design: Systematic Review
Device or Material: HA fillers included Restylane (Galderma Laboratories, Lausanne, Switzerland), Teosyal Pure
(Teoxane Laboratories, Geneva, Switzerland), and Juvederm Ultra XC (Allergan, Dublin, Ireland); and
Juvederm Voluma XC, Perlane, Belotero Balance, Redensity II Eyes, unspecified CL-HA, Uma Jeunesse,
Matrifill, and Juvederm Volbella (manufacturers NR) vs. calcium hydroxyapatite (CaHa), autologous
fibroblast and keratin gel (AFKG), and collagen-based fillers
Contact Duration: 1 month, and >6 months
Dose: Volume per tear trough: ranged from 0.2 to 0.58 mL, Average Volume HA per patient was 1.16mL
Frequency/Duration: Single administration
Response: Discomfort, Ecchymosis, Edema, Erythema, Fluid collection, Infection, , Itching, Lumpiness, Migraine,
Migration, Tyndall effect
Patient characteristics (gender, mean age): 28 studies; 1956 patients; 1535 (78.5%) of the patients were injected
with hyaluronic acid filler; patient ages ranged from 18 to 89 years with a mean of 44.9 years; large female
majority (1456/1956, 74.4%)
Number per group: 1535 HA (839 Restylane, 151 Teosyal Pure, 150 Juvederm Ultra XC, 101 Juvederm Voluma XC,
100 Perlane, 52 Belotero Balance, 52 Redensity II Eyes, 40 unspecified CL-HA, 22 Uma Jeunesse, 18
Matrifill, 10 Juvederm Volbella), 376 CaHa, 35 AFKG, and 10 collagen-based
Observed adverse effects: For HA: Ecchymosis 125 (14.01); Erythema 33 (2.15); Edema 172 (11.21); Lumpiness 127
(8.27); Tyndall effect 88 (5.41); Product migration 30 (1.95); Hyaluronidase 77 (5.02); Infection 2 (0.13);
Fluid collection 18 (1.17); Itching 5 (0.33); Migraine 1 (0.07); Discomfort 16 (1.04) Overall complication
rates were significantly different between HA and non-HA fillers (p <0.0001), but the clinical significance is
unclear: HA: 50.75%; CaHa, 19.95%; AFKG, 11.43%; and collagen-based filler, 90%. Prolonged edema was
more likely to occur among patients receiving HA injections than in patients injected with the other materials
(P < 0.00001). The Tyndall effect and lumpiness were only reported in the HA group. Thirty of 31 patients
who were noted to have product migration were in the HA group. However, the number of patients who
received AFKG or collagen-based filler was very low, so for these fillers some complications may not have
been captured simply due to the low patient numbers.
Timing of adverse effects: NR
Factors that predict response: Patients on whom cannulas were used were less likely to develop ecchymosis; use of
sharp needle tips may result in more traumatic injury to the orbicularis, regional vasculature, and lymphatic
structures.
Source Citation: Kumar et al. 2021
42

Material Performance Study - Hyaluronic Acid
|
98
Study Design: Systematic Review
Device or Material: HA fillers included Teosyal® PureSense Ultra Deep (Teoxane Lab, Switzerland), Juvéderm
VOLUMA (Allergan plc, Dublin, Ireland), YVOIRE volume plus (LG Life Sciences, Seoul, Korea), VYC−20 or
VYC−17.5 from the Vycross range of HA-based products (Allergan, Dublin, Ireland), and 1 of 5 brands of HA
fillers used in some studies – Allergan, Dublin, Ireland; NyumaPharma, Arona, Italy; Merz Aesthetics,
Raleigh, NC; Neauvia, Lugano, Switzerland; and Teoxane, Geneva, Switzerland
Contact Duration: NR
Dose: Different formulations of hyaluronic acid filler were used among the studies with a volume of injection ranging
from 0.1 to 1.5 ml
Frequency/Duration: Single administration
Response: Bruising, Erythema, Hematoma, Post-injection pain, Transient edema, Vascular impairments
Patient characteristics (gender, mean age): 11 studies; 1101 patients; majority female; ranging from 18 to 68 years
of age
Number per group: Ranged from 29 patients to 280 patients across 11 studies
Observed adverse effects: Transient edema and erythema, post-injection pain, and bruising were some temporary
complications. Rare complications that were reported were vascular impairments and hematoma
Timing of adverse effects: Varied by study
Factors that predict response: Different formulations of HA filler were used among the studies with a volume of
injection ranging from 0.1 to 1.5 ml. Various techniques were used for the purpose of injection.
Source Citation: Stefura et al. 2021
43
Study Design: Systematic Review
Device or Material: HA fillers included Aesthefill, Belotero Basic/Balance, BioHyalux, biphasic HA filler (NASHA),
Captique, CPMHA, Dermalax implant plus, Dermicol-P35, DGE, DHF-001, Elravie, Emervel Classic, HA (Titan)
+ Infrared, HA-biphasic, HA-G-monophasic, HA-monophasic, HA-P-biphasic, Hylaform/Hyalform Plus, hylan
B plus, Juvederm 30/Ultra 3/Ultra Plus, Ultra, Koken, Matrifill, Neuramis, Perlane PER, Perlane-L, Perlane—
LGP, PP-501-A/B, Prevelle SILK, Puragen, Restylane, Restylane Defyne, Restylane Refyne, Restylane Sub-Q,
Revanesse Versa, Teosyal, Terafill, Uma Jeunesse Classic, Uma Jeunesse Ultra, Uma Jeunesse, VYC-17.5L
(HA+lidocaine), Zyplast versus Ial System Duo, polycaprolactone (PCL), porcine collagen, bovine collagen,
CaHA (Radiesse) + lidocaine, CaHA (Radiesse), human-based collagen (Cosmoplast)
Contact Duration: Up to 1 year
Dose: Mean injected volume varied by study from a low of 0.62 mL to a high of 2.4 mL
Frequency/Duration: Single administration
Response: Redness, Bruising, Swelling, Pruritus, Skin induration, Tenderness, Skin discoloration, Pain, Nodulus,
Hematoma, Infection, Vascular adverse events, Migration, Numbness, Lumpiness
Patient characteristics (gender, mean age): 51 studies with a total of 4,097 patients in multiple countries; 35 studies
including 3388 patients were conducted in multiple centers, and 16 single-center studies including 709
patients. The quality of the analyzed studies, according to the Cochrane RoB tool, is low. All studies
evaluated dermal filler injections in nasolabial folds. Mean age range 40 to 56 years.
Number per group: HA fillers (n=3623), non-HA fillers (n=474)
Observed adverse effects: Redness, bruising, swelling, pruritus, skin induration, tenderness, skin discoloration, pain,
nodulus, hematoma, infection, vascular adverse events, migration, numbness, and lumpiness. Most

Material Performance Study - Hyaluronic Acid
|
99
common events included lumpiness, tenderness, swelling and bruising. Overall complication rates were 0.59
(95% CI: 0.46–0.72) for all HA fillers, 0.59 (95% CI: 0.36–0.8) for monophasic HA fillers, 0.6 (95% CI:
0.43–0.76) for biphasic HA fillers. For non-HA fillers, complication rates were 0.4 (95% CI: 0.12–0.7) for all
Collagen fillers, 0.82 (95% CI: 0.69–0.93) for Mesoglow, and 0.88 (95% CI: 0.72–0.99) for IAL-systems.
Timing of adverse effects: Up to 1 year
Factors that predict response: Variation in injection techniques including injected volume, HA concentration, depth of
injection, needle, eventual touch-up injections and the method of injection; Risk of vascular occlusion seems
higher in patients with history of cosmetic rhinoplasty; Vascular adverse events associated with potential
skin necrosis can occur.
Source Citation: Chung et al. 2020
44
Study Design: Systematic Review
Device or Material: HA dermal fillers included Restylane (Q-Med AB, Galderma, Uppsala, Sweden), Juvederm
(Allergen, Irvine, CA) and Belotero (Merz, Greensboro, NC)
Contact Duration: NR
Dose: NR
Frequency/Duration: Single administration
Response: Delayed inflammatory response, Delayed-type hypersensitivity (DTH)
Patient characteristics (gender, mean age): 35 prospective studies (4043 patients); 2 prospective studies with
histological examinations (438 patients); 7 retrospective studies (size of population ranged from 320 to
406,000); 15 case studies (20 patients)
Number per group: Restylane (26 articles), Juvéderm (12 articles), and Belotero (1 article)
Observed adverse effects: Delayed inflammatory response; DTH. The incidence of delayed inflammatory reactions
calculated from the prospective studies was 1.1% per year, and that of possible DTH reaction was 0.06%
per year. Most retrospective studies estimated a percentage of delayed inflammatory reactions of less than
1% in 1 to 5.5 years. The incidence of DTH reaction would be lower than that. Among all the DTH cases
reported, only about 5% of them were proven to be genuine DTH reactions.
Timing of adverse effects: NR
Factors that predict response: Previous adverse reactions to HA implants; Pretreatment skin tests are useful for
preventing potential DTH reaction; adverse events after the injection of non-FDA-approved fillers may be
higher than those seen after utilizing the FDA-approved products.
Source Citation: Kapoor et al. 2020
45
Study Design: Systematic review
Device or Material: HA dermal fillers included Bellast, Belotero, Hyacorp Modelis, Restylane
Contact Duration: NR
Dose: Data show that in a vast majority of cases (n = 17/19; 89.5%), the volume of HA filler used was less than or
equal to 2.0 ml
Frequency/Duration: Single administration
Response: Vision loss, CNS involvement, Ptosis, Ophthalmoplegia, Exotropia

Material Performance Study - Hyaluronic Acid
|
100
Patient characteristics (gender, mean age): 26 articles reporting 44 cases of vision loss; female (n=40; 90.9%); male
(n=4; 9.1%); age range 20-61 years
Number per group: 44
Observed adverse effects: Partial and complete loss of vision; periocular changes including ptosis, ophthalmoplegia
and exotropia; 8/44 patients had CNS involvement in the form of brain infarcts or hemorrhage.
Timing of adverse effects: Vision loss symptoms were almost always immediate; CNS involvement was 7 and 9 hours
after injection
Factors that predict response: Nose, glabella, and forehead were the common dangerous areas for injections; HA
filler volumes as low as 0.2 ml can cause permanent, complete vision loss; ‘Partial’ vision loss after HA filler
was found to have a better prognosis than the ‘complete’ vision loss. Branch retinal artery occlusion was
found to be the most favorable pattern for better prognosis after HA filler-related vision loss, while central
retinal artery occlusion and ophthalmic artery occlusion patterns were associated with the poorest
prognosis.
Source Citation: Stojanovi et al. 2019
46
Study Design: Systematic review
Device or Material: HA dermal fillers included Belotero, Emervel, Hyamax Kiss, Juvederm Ultra, Juvederm Ultra Plus,
Juvederm Volbella, Perfectha Derm, Perlane, Restylane, Restylane Kysse
Contact Duration: 1 month to 6 years
Dose: NR
Frequency/Duration: Single administration
Response: Bruising, Contusion, Itching, Pain, Redness, Swelling
Patient characteristics (gender, mean age): 22 studies of 3965 subjects; average age between 40 and 55 years;
majority of patients were women. All received HA fillers to enhance lip fullness.
Number per group: Sample size ranged from 10 to 400 patients.
Observed adverse effects: Local reactions at the injection sites (swelling, contusion, bruising, pain, redness, and
itching); serious AEs are uncommon. Most resolved in less than 60 days (majority in 2 weeks)
Timing of adverse effects: NR
Factors that predict response: Number and volume of HA‐based gel injections, the nature of the product injected as
well as possible individual factors
Source Citation: Beer et al. 2021
47
Study Design: Multicenter RCT
Device or Material: HA injectable filler, VYC-20L (Juvederm Voluma XC)
Contact Duration: Visits occurred at months 1, 3, 6, 9, and 12 after the last treatment
Dose: Median total initial injection volume was 2.2 mL (range, 0.7–4.0 mL) for the treatment group (initial treatment
and touch-up combined) and 2.8 mL (range, 1.3–4.0 mL) for the treated control group (initial treatment and
touch-up combined) Median total injection volume for repeat treatment was 1.2 mL (range, 0.2–4.0 mL)
Frequency/Duration: 1 or 2 injections

Material Performance Study - Hyaluronic Acid
|
101
Response: Injection site abscess, Gingival pain, Cystic acne, injection site cellulitis, injection site inflammation,
injection site induration
Patient characteristics (gender, mean age): Enrolled participants desired chin augmentation. The majority were
women (88.5%) and White (81.8%), with a median age at study entry of 52 years (range, 22–80) and
mean body mass index of 25.0 kg/m
2
. Fitzpatrick skin types were I/II (34.9%), III/IV (52.1%), and V/VI
(13.0%).
Number per group: 192 participants were randomized resulting in 144 in the treatment group and 48 in the control
group (which received HA injection 6 months after the treatment group).
Observed adverse effects: Common treatment-related AEs included injection site abscess, gingival pain, cystic acne,
injection site cellulitis, and injection site inflammation. For initial/touch-up treatment, 14 treated participants
(7.7%) had 20 treatment-related AEs while 3 (4.1%) had 7 treatment-related AEs after repeat treatment.
Most treatment-related AEs were mild or moderate in severity. For initial/touch-up treatment, 2.7% (5/182)
of participants had mild treatment-related AEs and 4.4% (8/182) had moderate AEs. For repeat treatment,
4.1% (3/74) of participants had mild and 1.4% (1/74) moderate AEs. Two participants (1.1%) had 3 severe
treatment-related AEs, including injection site inflammation and cellulitis in one participant and injection site
induration in another participant. Overall, 167 treated participants (92.3%) reported at least 1 ISR after
initial treatment, 86 (82.7%) after touch-up treatment, and 55 (75.3%) after repeat treatment. The most
frequently reported ISRs after initial treatment included tenderness to touch (81.8%), firmness (75.1%),
and swelling (68.5%). Similar results were seen after repeat treatment, where the most common ISRs were
also tenderness to touch (71.2%), firmness (69.9%), and swelling (58.9%).
Timing of adverse effects: Injection site abscess (onset 6 days), gingival pain (onset 1 day), acne cystic (onset 6
days), injection site cellulitis (onset 7 days), and injection site inflammation (onset 7 days)
Factors that predict response: A lower incidence of ISRs was observed for injections with a cannula than without a
cannula after initial touch-up and repeat treatments. There was an overall decrease in the number of AEs with repeat
treatment compared with initial/touch-up treatment. One possibility is the lower volume injected during repeat
treatment.
Source Citation: Cheon et al. 2021
48
Study Design: Multicenter RCT (split-face design)
Device or Material: DIVAVIVA medium and Restylane Perlane Lidocaine
Contact Duration: Study 1: 24 weeks from baseline. Study 2 (Extension): 25 to 52 weeks
Dose: NR
Frequency/Duration: Single administration
Response: Bruise, Edema, Erythema, Pain, Pruritis
Patient characteristics (gender, mean age): Majority female, 107 subjects with bilateral symmetric moderate to
severe nasolabial folds with grade 3 or 4 on a Wrinkle Severity Rating Scale. In the full analysis set, age
ranged from 32 to 65 years.
Number per group: Study 1: 107 enrolled/106 completed; Study 2: 87 enrolled/82 completed
Observed adverse effects: Study 1: # of injection site-related adverse events (pain, edema, bruise, pruritis, and
erythema) was 40 in DIVAVIVA group, 36 in Restylane Perlane Lidocaine group, there were no severe
injection site-related adverse events. They were all mild. Study 2: The number of adverse events occurring
during extension study was 13 cases. All adverse events were mild. One case, injection site mass, was
considered to be possibly related to the device.

Material Performance Study - Hyaluronic Acid
|
102
Timing of adverse effects: Study 1: All cases recovered within 10 days without treatments. Study 2: Cases
recovered during the extension study.
Factors that predict response: Not reported
Source Citation: Lee et al. 2021
62
Study Design: Single center retrospective single-arm study
Device or Material: Facial fillers (not specified)
Contact Duration: Followed up for 6 months or longer
Dose: NR
Frequency/Duration: Single administration
Response: Purpura/blister, Ptosis/ophthalmoplegia, Subconjunctival hemorrhage, Focal skin discoloration, Purpura
Patient characteristics (gender, mean age): 10 consecutive patients who presented with occluded periorbital vessels
after facial injection.
Number per group: 7 injected with HA, 1 with collagen, 1 with poly-L-lactic acid, and 1 with a local anesthetic of 2%
lidocaine with 1:100,000 epinephrine.
Observed adverse effects: Purpura/blister or purpura occurred in 6/7 (85%) patients with HA. Ptosis/ophthalmoplegia
or ophthalmoplegia occurred in 4/7 (57%). Additional complications included subconjunctival hemorrhage
(5) and facial discoloration (1).
Timing of adverse effects: Time to symptom after injection included immediately (n = 6), within 30 min (n = 2),
within 2 h (n = 1), and within 2 days (n = 1). Time to presenting after vascular accident included within 6 h
(n = 5), 1 day (n = 1), 2 days (n = 2), and 3 days (n = 2).
Factors that predict response: Clinical features are diverse according to the site and extent of vascular occlusion and
injection materials. Visual prognosis was associated with the site of vascular occlusion and initial visual
acuity.
Source Citation: Munavalli et al. 2021
63
Study Design: Case series of 4 cases
Device or Material: Types of HA fillers included Restylane Lyft, Restylane L, Juvederm Volbella, Juvederm Voluma,
and Juvederm Ultra
Contact Duration: 1 to 2.5 years
Dose: NR
Frequency/Duration: Varied by case
Response: Edema, Erythema, Swelling, Tenderness
Patient characteristics (gender, mean age): Four (4) female patients ages 50, 51, 36, and 43. All had h/o receiving
HA filler injections. (HA filler for Case 4 not identified). Timing of last HA filler injections varied and was
identified as previous 12 months, previous 18 months, 2019, and 2.5 years ago.Confirmed or potential
exposure to COVID-19 varied and was identified as positive COVID-19 diagnosis, receipt of first blinded
injection in an mRNA-1273 clinical phase III trial, injection of first dose of Moderna vaccine, and injection of
2
nd
dose of Pfizer vaccine.
Number per group: 4 cases/4 patients

Material Performance Study - Hyaluronic Acid
|
103
Observed adverse effects: Case 1: severe periorbital swelling, followed by worsening facial edema, erythema, and
tenderness. Case 2: facial edema, erythema, tenderness and pain
Note: Case 2 was eliminated from the
study. Two subsequent COVID-19 antibody tests were negative and unblinding revealed that this patient
received the saline injection.
Case 3: Increased tenderness in the right tear trough, followed by worsening
of unilateral infraorbital edema and development of perioral edema. Also noted that the face remained
persistently swollen in areas of previous filler injection. Case 4: Mild tenderness underneath the right eye,
followed by swelling under the left eye
Timing of adverse effects: Case 1: Onset 2 weeks after positive COVID-19 diagnosis. Case 2: Onset 8 days after
blinded injection. Case 3: Onset 12 h after vaccination. Case 4: Onset 24-hours after 2
nd
vaccination
Factors that predict response: The authors hypothesize that a COVID-19 spike protein triggers inflammatory
reactions to dermal HA filler.
Source Citation: Humphrey et al. 2020
64
Study Design: Retrospective single-arm study
Device or Material: Juvederm Voluma (HA-V)
Contact Duration: 9-year period
Dose: Average cumulative volume before the first reported DAE was 5.1 mL (clinic 1) and 4.5 mL (clinic 2), for a
combined cumulative volume of 5.0 mL.
Frequency/Duration: Single administration
Response: Erythema, Nodule formation, Pain, Swelling, Tenderness, Warmth
Patient characteristics (gender, mean age): 9324 treatments in 4500 patients (3908 women, 592 men)
Number per group: 44 patients (1 man, 43 women) aged 27 to 79 years (average, 59 years) who experienced DAEs,
for a combined incidence rate of 0.98% per patient, 0.47% per treatment, and 0.23% per 1-mL syringe
Observed adverse effects: Delayed swelling and nodule formation (29 each) were the most common reactions, along
with erythema, warmth, and tenderness or pain. Two patients experienced a single DAE comprising more
than 1 episode.
Timing of adverse effects: The median time to onset of DAE from the most recent injection of HA-V was 4 months
(range, 1-13 months). Of 22 patients who received multiple injections of HA-V, the median time of onset
from first injection was 15.5 months (range, 3-65 months).
Factors that predict response: Patients who experienced DAEs received 0.5 to 13.3 mL of HA-V in multiple locations,
most commonly in the mid and lower face. Nearly half of the patients (21/44) received more than 1
treatment with HA-V before DAE onset. The average cumulative volume of HA-V before the first reported
DAE was 5.1 mL (clinic 1) and 4.5 mL (clinic 2), for a combined cumulative volume of 5.0 mL. Of 44 cases
of DAEs, 41 occurred in patients with HA-V that had been blended/admixed with variable volumes of
lidocaine with epinephrine and/or normal saline. More than half of the reactions (23/44) occurred between
October and January. A review of cases to identify potential immunologic triggers showed that 15 of 44
(34.1%) had an identifiable immunologic stimulus such as flu-like illness, infection, or dental procedure
immediately before DAE onset.
Source Citation: Kassir et al. 2020
65
Study Design: Retrospective single-arm study
Device or Material: Fillers for non-surgical rhinoplasty including HA (Restylane) and Calcium hydroxyapatite
Contact Duration: NR

Material Performance Study - Hyaluronic Acid
|
104
Dose: Average injection volume varied by location: Nasal tip 0.1–0.2cc, Columellar base 0.1–0.2cc, Dorsum 0.1–
0.4cc, Radix 0.1–0.2cc
Frequency/Duration: Single administration
Response: Persistent redness
Patient characteristics (gender, mean age): Since 2006, 2130 patients older than 18 who underwent non-surgical
rhinoplasty; 2023 patients were female (95%), and 107 were male (5%)
Number per group: Yearly distribution began with 35 cases in 2006 and increased each year with the highest number
of 342 cases in 2019, the last year of the study. Each of the nose regions was injected in the following
percentage of patients: Tip 95%, Columella 58%, Dorsum 83%, and Radix 62%.
Observed adverse effects: Two percent of patients had persistent tip redness which was observed suspecting
possible necrosis. However, this recovered spontaneously, suggesting external vascular compression in the
tip rather than intraarterial occlusion. There was no skin necrosis or ocular complications.
Timing of adverse effects: The duration of redness lasted from 5 to 60 min.
Factors that predict response: NR
Source Citation: Ogilvie et al. 2020
49
Study Design: RCT
Device or Material: HA injectable filler VYC-25L (Juvederm Volux)
Contact Duration: Month 1 and 3, 12, 18 and 1 month after retreatment
Dose: Median range volume injected, mL Treatment group (Initial/Touchup): 3.43 (1.2-4.1), n = 90; After repeat
treatment: 3.00 (0.5-5.0), n = 65 Control group (Initial/Touchup): 3.55 (1.7-4.0), n = 29; After repeat
treatment: 2.50 (0.8-4.0), n = 24
Frequency/Duration: Single administration
Response: Bruising, Firmness, Injection-site pain, Swelling, Tenderness, Unclear pronunciation
Patient characteristics (gender, mean age): The mean age was 46.2 [13.3] years (range, 20-75 years), most subjects
were female (92%, n = 110; the remaining 8% were men, n = 10), and the population was predominantly
white (85.8%). At baseline, the mean glabella-subnasale-pogonion angles were 160.6° [4.2°] for the Volux
group and 161.3° [2.8°] for controls.
Number per group: A total of 120 subjects were randomized (90 in the Volux group, 30 in the control group); of
these, 119 subjects received initial/touch-up Volux treatment (90 Volux, 29 control) and 89 subjects (65
Volux, 24 control) received repeat treatment.
Observed adverse effects: The mean pain score as assessed by all treated subjects immediately after initial and
touch-up treatment (n = 119) was 2.6 [1.8]; almost identical to that after repeat treatment (n = 87; 2.6
[2.2]). Most subjects who received treatment reported at least 1 ISR; 96.6% for initial and touch-up
treatment combined and 95.5% for repeat treatment. The most commonly reported ISRs with initial/touch-
up and repeat treatments were firmness (95.8% vs 94.4%) and tenderness to touch (95.8% vs 94.4%),
respectively. Severe ISRs of bruising and firmness occurred at higher-than-expected rates after initial/touch-
up treatment, but at typical rates for a HA soft tissue filler after repeat treatment (statistical significance
testing was not performed). Most ISRs had a duration of 1 week. A total of 53 TEAEs occurred in 18
(20.2%) subjects. All TEAEs were at the injection site. Three TEAEs were reported more than 1 year after
initial/touch-up treatments; 2 subjects reported swelling and 1 subject reported pain. All 3 events were of
mild or moderate severity and resolved within 2 days without treatment or sequelae. The most common
TEAEs following repeat treatment were the same as TEAEs following initial/touch-up treatments, although
rates were more variable: injection site mass (14.6% vs 21.8%), injection site induration (13.5% vs

Material Performance Study - Hyaluronic Acid
|
105
12.6%), and injection site pain (7.9% vs 12.6%), respectively. Nearly all TEAEs were related to ongoing
ISRs. No serious TEAEs (related or unrelated to treatment) were reported, and no subject discontinued the
study because of a TEAE after repeat treatment. Four subjects experienced speech disorder (specifically,
unclear pronunciation) following initial and touch-up treatments, and 1 subject experienced unclear
pronunciation following repeat treatment.
Timing of adverse effects: Pain: At initial treatment and touch-up treatment ISRs: At initial treatment, touch-up
treatment, and repeat treatment TEAEs: Reported more than 1 year after initial/touch-up treatments and
repeat treatments
Factors that predict response: Volux was chosen for this study because it has the highest concentration of HA (25
mg/mL), which was anticipated to provide the strong volumizing and lift properties necessary to sculpt,
shape, and contour the high-mobility chin and jaw areas.
Source Citation: Wortsman et al. 2020
58
Study Design: Prospective comparative study
Device or Material: Cosmetic fillers (CFs): HA (not specified), silicone oil, polymethylmethacrylate, polycaprolactone,
calcium hydroxyapatite, and poly acrylamide
Contact Duration: ≥4 months after the last filler injection in >50%
Dose: NR
Frequency/Duration: Administration in >one facial location or in >two facial locations
Response: Ultrasound alterations of the lacrimal, parotid, and submandibular glands after filler injection
Patient characteristics (gender, mean age): 63 female patients (mean age: 53 years; SD 12.9) with h/o cosmetic filler
injection in the nasolabial, periocular, or labial-peribuccual regions of the face.
Number per group: Location of fillers % (CI 95%): Nasolabial 85.7 (77–94.3), Periocular 66.7 (55.1–78.3),
Labial-peribuccal 82.5 (73.1–91.9) Distribution of fillers % (CI 95%): >One facial location 85.7 (77.1–
94.3), >Two facial locations 71.4 (60.3–82.6) Fillers identified in the sample: HA in 86%, silicone oil in
48%, polymethylmethacrylate in 40%, polycaprolactone in 19%, calcium hydroxyapatite in 4.8%, and
polyacrylamide in 1.6% of cases
Observed adverse effects: Ultrasound Alterations of the Glands % (95% CI): Lacrimal gland echostructure of
the parenchyma 58.7 (46.5–70.9), Lacrimal gland hypervascularity 38.1 (26.1–50.1), Parotid gland
echostructure of the parenchyma 87.3 (79.1–95.5), Parotid gland hypervascularity 14.3 (5.7–22.9),
Submandibular gland echostructure of the parenchyma 88.9 (81.1–96.6), Submandibular gland
hypervascularity 34.9 (23.1–46.7).
Timing of adverse effects: Ultrasound examination took place ≥4 months after the last filler injection in most cases
(≥ 50%)
Factors that predict response: The presence of filler deposits close to the glands increased the chance of alterations
in these glands. HA, by itself or with hyaluronidase, presented a high percentage of abnormalities in the
glands. The mix of HA and other CFs had 20.8 more times the risk of alterations in the parotid gland (CI
1.8–240.9) compared to HA alone. The presence of more than 2 types of CFs and 2 or multiple facial
locations presented a higher risk of abnormality of the glands.
Source Citation: Zhang et al. 2020
66
Study Design: Retrospective single-arm study

Material Performance Study - Hyaluronic Acid
|
106
Device or Material: HA fillers including Juvina, Allaxin, Perlane, Juvederm, Restylane, Juvederm Voluma XC, Matridex
(HA and dextranomer), Juvederm Ultra
Contact Duration: 10 year period
Dose: NR
Frequency/Duration: Single administration
Response: Delayed granuloma reactions in the orofacial region following HA filler injections
Patient characteristics (gender, mean age): All patients were female, with a mean age of 38.5 years, who had
developed delayed granulomatous reactions after receiving HA injections and underwent surgical resections
in the authors’ hospital.
Number per group: 11 patients (8 had been injected with HA fillers).
Observed adverse effects: Palpable nodules with/without growth and with/without pain
Timing of adverse effects: The nodules had developed 3 months to 6 years before surgical resections, and roughly 3
to 10 years following filler injection.
Factors that predict response: Different brands of dermal fillers, HA and non-HA, were injected into the same
anatomical location. In one case a non-absorbable non-HA filler was injected.
Source Citation: Dai et al. 2019
50
Study Design: RCT (split-face design)
Device or Material: Princess® VOLUME (PV) vs Restylane
Contact Duration: 24 weeks
Dose: 1 mL median injection volume
Frequency/Duration: Most received 1 injection (only 2 patients had a follow-up injection)
Response: Injection site hemorrhage, Injection site nodules, Pain, Swelling
Patient characteristics (gender, mean age): 96.67% female, mean age of 43 years (range 29–64 years)
Number per group: 120 patients total (120 received Restylane in one nasolabial fold and PV in the other), 115
patients completed the study.
Observed adverse effects: 58 subjects (48.33%) reported at least one AE for PV, and 53 (44.17%) reported at least
one AE for Restylane. Overall, the most frequently reported AEs for PV and Restylane were swelling
(30.83% and 29.17%, respectively) and pain (21.67% and 23.33%, respectively). Although more patients in
the PV group had injection site nodules (16.67% vs 10.83%), the difference was not statistically significant.
Overall, the incidence rates of AEs were similar in both groups. No severe AEs were reported.
Timing of adverse effects: Most AEs lasted for 14 days or less in 98.28% and 96.23% of subjects who had a
treatment site response to PV or Restylane, respectively.
Factors that predict response: NR
Source Citation: Kaufman-Janette et al. 2019
51
Study Design: RCT (split‐face design)
Device or Material: RHA
®
4 (RHA4) and Restylane
®
Lyft
Contact Duration: 1 year up to 15 months

Material Performance Study - Hyaluronic Acid
|
107
Dose: Volumes (initial + touch up): 1.79 (±0.87) mL for RHA4 vs 1.75 (±0.90) mL for Lyft, (P = .48, NS).
Frequency/Duration: Single administration
Response: Bruising, Discoloration, Firmness, Itching, Lumps/bumps, Pain, Redness, Swelling, Tenderness
Patient characteristics (gender, mean age): The per-protocol (PP) analysis group included 81 women and 7 men and
had a mean (±SD) age of 57 (±9) years. Fitzpatrick skin types: 61.4% of subjects had skin types I‐ to‐III
and 38.6% had skin types IV‐VI.
Number per group: 88 subjects were included in the PP population; 18 subjects were in the untreated control group.
All subjects who received treatment were considered for the safety analysis: n = 120 for the SAFT
population.
Observed adverse effects: CTRs: bruising, discoloration, firmness, itching, lump/bumps, pain, redness, swelling, and
tenderness. TRAEs: Frequencies of TRAEs were consistent with expected incidence rates and similar
between treatment groups. Seventy‐one (59%) and 62 (52%) subjects experienced a TRAE related to RHA4
and Lyft, respectively. All TRAEs were mild‐to‐moderate (no severe TRAE), and 334 of 364 events reported
(92%) were administration site conditions. Overall, most frequent TRAEs were injection lumps/bumps (n =
55/120, 45.8% of subjects), injection site firmness (n = 49/120, 40.8%), injection site swelling (n = 27/120,
22.5%), and tenderness (n = 21/120, 17.5%). There were no reports of late‐onset TRAEs or granulomas
with either filler.
Timing of adverse effects: CTRs: Injection pain was virtually absent 5 minutes postinjection, and there were no
clinically meaningful differences between devices regarding pain. Proportions of subjects experiencing at
least one CTR were comparable between treatment groups and the majority had resolved by Day 14.
TRAEs: Any CTR extending past 14 days was recorded as a TRAE. No late‐onset TRAEs or granulomas were
reported. Three subjects experienced a serious AE (arthralgia, diverticulitis, and lung infection), but none
was deemed to be related to the study treatment.
Factors that predict response: The global incidence of TRAEs was higher in subjects with Fitzpatrick skin types I‐III
(n = 48, 71.6%) than in those with types IV‐VI (n = 29, 54.7%).
Source Citation: Li et al. 2019
52
Study Design: RCT (split-face design)
Device or Material: Restylane Lyft vs Restylane
Contact Duration: 12 months
Dose: 1.1 mL mean injection volume
Frequency/Duration: 1 or 2 injections
Response: Transient bruising
Patient characteristics (gender, mean age): 98% female, mean age 43.6 ± 8.1 years
Number per group: 100 per group (each cheek randomized to a different treatment) (100 total patients, 95
completed the study)
Observed adverse effects: Two subjects (2%) experienced AEs related to the study product and/or injection
procedure: transient bruising after treatment (mild intensity with Restylane and moderate intensity with
Restylane Lyft). Both AEs spontaneously resolved within the following week, and no treatment‐related
serious AEs were reported.
Timing of adverse effects: Within 1 week of injection
Factors that predict response: NR

Material Performance Study - Hyaluronic Acid
|
108
Source Citation: Sadeghpour et al. 2019
59
Study Design: Retrospective comparative study
Device or Material: Juvederm Volbella (VOB), Juvederm Vollure (VLR), Juvederm Voluma (VOL), Restylane Silk
Contact Duration: Up to 54 weeks
Dose: 0.5 mL per injection
Frequency/Duration: 1 to 3 injections
Response: Delayed-onset nodules
Patient characteristics (gender, mean age): Across groups, 91.1% to 97.6% were female, mean age ranged from
58.6 to 62.7 years
Number per group: 315 VOL, 219 VLR, 495 VOB, 385 Restylane Silk
Observed adverse effects: Five patients (1.0%) developed delayed nodules after VOB injection. No nodules were
observed in patients who received VLR or VOL. One patient (0.25%) developed delayed nodules after
Restylane Silk injection. All nodules were treated successfully using a combination of intralesional
triamcinolone and hyaluronidase. No patient developed systemic symptoms.
Timing of adverse effects: 20 to 54 weeks after first VOB injection, 6 to 37 weeks after last VOB injection.
Factors that predict response: NR
Source Citation: Turkmani et al. 2019
67
Study Design: Retrospective single-arm study
Device or Material: Several HA dermal fillers (Belotero, Hylaform, Juvederm, Restylane, Surgiderm, Teosyal)
Contact Duration: 2 to 10 months
Dose: NR
Frequency/Duration: 2 to 8 injections
Response: Delayed hypersensitivity reaction to HA fillers following influenza-like illness
Patient characteristics (gender, mean age): 100% female, age range 22 to 65 years
Number per group: 14 females who experienced delayed hypersensitivity reactions
Observed adverse effects: All 14 patients experienced delayed hypersensitivity reactions at HA filler sites 3-5 days
following an influenza-like illness. The most common sites of the reactions were the cheeks (n=9), tear
trough (n=8), and lips (n=3). Patients were treated with oral prednisolone 20–30 mg or Methyl
prednisolone 16–24 mg daily for 5 days, followed by tempering of the dose for another 5 days. After a 2
week follow up, there was a complete resolution of the symptoms in 10 patients following the oral steroid
therapy. The remaining 4 patients still presented minimal swelling that was treated with hyaluronidase 1
month after the onset of symptoms.
Timing of adverse effects: 2 to 10 months following last filler injection.
Factors that predict response: NR
Source Citation: Ueland et al. 2019
60
Study Design: Prospective comparative study

Material Performance Study - Hyaluronic Acid
|
109
Device or Material: Juvederm Voluma vs autologous fat (AF)
Contact Duration: 24 months
Dose: Juvederm median injection volume 0.9 (0.2 to 2) mL, AF median volume 3.1 (0.5–9.6) mL
Frequency/Duration: 1 to 5 injections (median 1 for Juvederm, 2 for AF)
Response: Bruising, Disfiguring surface, Pain, Swelling
Patient characteristics (gender, mean age): 93% female, mean age 62 (range 33 to 77) years
Number per group: 17 patients were treated bilaterally and 12 unilaterally (5 received Juvederm and 7 AF) for
temporal hollowing. Patients treated bilaterally received injections of Juvederm in the right temple and AF in
the left temple. Additional injections were given when needed at follow-up after 6, 12, 18, and 24 months.
Observed adverse effects:
Early complications
included bruising, pain and swelling which did not differ between
Juvederm and AF injection sites. Juvederm had more cases of irregular surface (5/22) compared to AF
(1/24).
Late complications
: Swelling occurred in 3/22 Juvederm sites and no AF sites; it resolved within 12
months. Subcutaneous scarring occurred only in 5/24 AF sites (no Juvederm sites developed this event).
Abdominal pain occurred in 2 patients due to AF harvest. Disfiguring surface occurred in 2/22 Juvederm
sites and 1/24 AF sites.
Timing of adverse effects: Early complications occurred within first 2 weeks of injection, late complications occurred
after 2 weeks and up to 12 months.
Factors that predict response: NR
Source Citation: Wu et al. 2019
53
Study Design: RCT (split-hand design, no- treatment control)
Device or Material: HA filler, Restylane® Vital (RESv)
Contact Duration: Injection on day 1, at 1 month, and at 2 months
Dose: 0.5-1 mL RESV per injection
Frequency/Duration: Single administration
Response: Bruising, Injection-site discoloration, Injection-site pain, Injection-site swelling, Redness, Swelling
Patient characteristics (gender, mean age): 96 females, 4 males; 49 years of age on average (ranging from 23 to 74
years); 93% were of Han Chinese background; 71% had Hand Grading Scale (HGS) score of 2 (moderate)
and the rest had a score of 3 (severe).
Number per group: 100 subjects enrolled, 7 withdrawn from study; Subjects were randomly assigned to have one
hand be treated and the opposite hand to no treatment.
Observed adverse effects: Local tolerability reactions included redness, bruising, and swelling. 27 treatment-related
AEs were reported by 12 (12%) subjects, and the most common were injection site swelling (6%), injection
site pain (4%), and injection site discoloration (4%). Most related AEs were mild or moderate.
Timing of adverse effects: Most AEs occurred at time of treatment. Five subjects reported related AEs with delayed
onset (>21 days after last treatment) after the third treatment, such as injection/implant site pruritus,
swelling, and discoloration.
Factors that predict response: Frequent hand washing was determined to be an occupational risk factor for
developing hand skin irritation associated with report of AEs with delayed onset.
Source Citation: Prager et al. 2017
54

Material Performance Study - Hyaluronic Acid
|
110
Study Design: RCT (split-face design)
Device or Material: Belotero Volume vs Juvederm Voluma
Contact Duration: 18 months
Dose: 2 mL per injection
Frequency/Duration: Single injection of each filler (different sides of the face)
Response: Swelling, Pain, Redness, Bruising, Firmness, Itching, Discoloration
Patient characteristics (gender, mean age): 96% female, mean age 50.3 years
Number per group: 46 patients total (each received a different injection on each side of their face)
Observed adverse effects: 39 and 40 patients reported at least 1 injection site reaction for Belotero and Juvederm,
respectively. Pain, redness, swelling, bruising, firmness/induration, itching and coloration/discoloration, did
not show a statistically significant difference between treatments. One case of headache was considered
possibly related to the injections.
Timing of adverse effects: Day 0 to 18 months.
Factors that predict response: NR
Source Citation: Weiss et al. 2016
55
Study Design: RCT (control group had no treatment)
Device or Material: Restylane Lyft
Contact Duration: 15 months
Dose: Mean 6.23 mL (range, 2.00– 14.00 mL) at initial injection, mean 3.80 mL (range, 0.30–10.10 mL) at the 12-
month retreatment.
Frequency/Duration: 1 to 2 injections
Response: Swelling, Pain, Hematoma, Infection, Late onset inflammation
Patient characteristics (gender, mean age): 92% female, mean age 52.9 ± 7.6 years
Number per group: 150 patients received midface injections of Restylane, 50 patients received no treatment
Observed adverse effects: TEAEs were more often related to injection procedure than to device and were mostly mild
and transient; the most common were implant site hematoma, implant site pain, and implant site swelling.
Four serious AEs in 2 subjects were considered to be related to the procedure or device; one subject
reported implant site inflammation (late onset inflammatory reactions) in both cheeks at separate times.
The other subject experienced implant site hematoma and implant site infection in the right cheek. All
events resolved.
Timing of adverse effects: Most events occurred within 1 day of injection.
Factors that predict response: NR
Source Citation: Beleznay et al. 2015
68
Study Design: Retrospective single-arm study
Device or Material: Juvederm Voluma
Contact Duration: Median 4 months
Material Performance Study - Hyaluronic Acid
|
111
Dose: Median 3 mL (range 1 to 12.3 mL)
Frequency/Duration: 1 or 2 injections
Response: Delayed-onset nodules
Patient characteristics (gender, mean age): Gender NR, mean age 54 years in cases.
Number per group: 23 cases of delayed-onset nodules out of 4,702 total Juvederm facial injections in 2,342 patients
Observed adverse effects: Delayed-onset nodules occurred in 0.5% of Juvederm injections. No systemic symptoms
were reported.
Timing of adverse effects: Median 4 months after injection, range 1 to 13 months. The median time to resolution of
the nodule's following treatment was 6 weeks (range, 1 to 36 weeks).
Factors that predict response: Nine of the 23 (39%) had an immunologic stimulus such as flu-like illness or dental
procedure immediately preceding the reaction.
Source Citation: Butterwick et al. 2015
56
Study Design: RCT
Device or Material: Juvederm Ultra XC vs Belotero Balance
Contact Duration: 6 months
Dose: Mean 1.25 mL
Frequency/Duration: 1 or 2 injections
Response: Swelling, Induration, Nodules, Bruising, Tyndall effect
Patient characteristics (gender, mean age): 99% female, mean age 58.2 ± 8.4 years
Number per group: 69 per group (138 total)
Observed adverse effects: Adverse events related to the procedure were reported for 10 (15%) subjects in each arm.
Severe injection site bruising was reported for 1 (1%) subject in each arm. Adverse events deemed related
to the device were reported for 3 (4%) subjects in the Juvederm arm (induration, swelling, nodules) and 1
(1%) subject in the Belotero arm (Tyndall effect). There were no statistically significant differences in
incidence or severity of injection site reactions.
Timing of adverse effects: One day to 6 months, most occurred within the first month.
Factors that predict response: NR
Source Citation: Sattler et al. 2014
57
Study Design: RCT
Device or Material: Juvederm Ultra 4 vs calcium hydroxylapatite (Radiesse)
Contact Duration: 12 months
Dose: Bolus per injection: 0.1 to 0.2 mL interdigitally
Frequency/Duration: 3 to 4 injections initially, some patients received 1 to 3 touch-up injections
Response: None
Patient characteristics (gender, mean age): 100% female, median age 56 (range 45 to 65 years)
Number per group: 37 patients (each hand received a different filler)

Material Performance Study - Hyaluronic Acid
|
112
Observed adverse effects: In total, 11 adverse events were documented in 6 patients. All adverse events were
related to the injection with calcium hydroxylapatite.
Timing of adverse effects: Within first 2 weeks of injection.
Factors that predict response: NR
Source Citation: Trignano et al. 2015
69
Study Design: Retrospective single-arm study
Device or Material: Macrolane
Contact Duration: 12 months
Dose: NR
Frequency/Duration: NR
Response: Intramammary and intramuscular cysts
Patient characteristics (gender, mean age): 100% female, mean age 35.75 years
Number per group: 20 patients who had experienced complications following Macrolane breast enhancement.
Observed adverse effects: All 20 patients presented with breast lumpiness and noted rapid, asymmetric losses in
breast volume. Six of these patients were concerned about existing breast ptosis that had been worsening
in association with the rapid resorption of HA. At least 85% of Macrolane filler was surgically removed from
the breasts of all patients.
Timing of adverse effects: Cysts developed within 12 months of injection.
Factors that predict response: NR
Source Citation: Sito et al. 2013
61
Study Design: Retrospective comparative study
Device or Material: HA (Macrolane) vs lipofilling
Contact Duration: Mean 24 months
Dose: Macrolane 30 to 40 mL, lipofilling unclear
Frequency/Duration: Single injection
Response: None
Patient characteristics (gender, mean age): 100% male, mean 33 years (range 26 to 42 years). All patients
underwent penile augmentation.
Number per group: Macrolane (n = 56), lipofilling (n = 27)
Observed adverse effects: No complications were observed in patients treated with Macrolane, but a granuloma
formed in 8 patients treated with lipofilling. The symptoms of 5 of these patients resolved spontaneously
within 6 months with the help of massage; 2 patients reported no relief of symptoms from massage, and fat
necrosis with progressive skin loss occurred in 1 patient after 20 days. That patient’s wounds were treated
conservatively with weekly dressing, and secondary healing occurred after 3 months.
Timing of adverse effects: Timing of granulomas unclear but average follow-up was 24 months.
Factors that predict response: NR

Material Performance Study - Hyaluronic Acid
|
113
Source Citation: Inglefield 2011
70
Study Design: Prospective single-arm study
Device or Material: Macrolane
Contact Duration: Mean 13.3 months
Dose: Mean 136 mL (range 20 to 200 mL)
Frequency/Duration: Single injection
Response: Capsular contraction, Early resorption, Infection, Removal of product
Patient characteristics (gender, mean age): 100% female, mean age 35 years (range: 19 to 62 years)
Number per group: 194 patients who received Macrolane for breast enhancement.
Observed adverse effects: Minor adverse events 12.4% (24 patients), major adverse events 8.7% (17 patients),
infection 0.5% (1 patient), capsular contraction 4.6% (9 patients), early resorption 3.1% (6 patients),
removal of product 0.5% (1 patient).
Timing of adverse effects: Most events occurred within the first 6 months
Factors that predict response: NR
Source Citation: Park et al. 2011
71
Study Design: Retrospective single-arm study
Device or Material: HA fillers (brand NR)
Contact Duration: Mean 5.3 months
Dose: NR
Frequency/Duration: NR
Response: Dyspigmentation, Inflammatory symptoms, Nodules, Tissue necrosis
Patient characteristics (gender, mean age): 82.1% female, mean age 33.7 ± 10.1 years
Number per group: 28 cases of HA filler-related facial complications
Observed adverse effects: 12 patients (42.9%) with a nodularity or palpable mass, 10 patients (35.7%) with
inflammatory symptoms such as swelling, tenderness and redness, three patients (10.7%) with tissue
necrosis, including one case of alar rim involvement and three patients (10.7%) with dyspigmentation.
Timing of adverse effects: Mean 5.3 months post-injection
Factors that predict response: NR
Systemic Response/ Toxicity
Source Citation: Alijotas-Reig et al. 2018
72
Study Design: Retrospective single-arm study
Device or Material: Dermal fillers/implants
Contact Duration: Onset 6 months or longer after bioimplant injection (mean 70.7 months)
Dose: NA
Material Performance Study - Hyaluronic Acid
|
114
Frequency/Duration: Single administration
Response: Autoimmune/ inflammatory syndrome induced by adjuvants (ASIA) - see list of specific symptoms under
observed adverse events
Patient characteristics (gender, mean age): 88.9% female, mean age NR
Number per group: 45 cases of patients suffering from late-onset, inflammatory, non-infectious adverse reactions
related to dermal fillers/implants. Only 7/45 patients received HA as the sole injected material. The
biomaterials in other cases were: polyalkylamide, poly-L-lactic acid, HA plus methacrylate, silicone medical
grade, and unknown filler.
Observed adverse effects:
Symptoms
: Of the 7 cases who received HA alone, the following symptoms were reported:
4 myalgia, 6 arthralgia/arthritis, 6 fatigue, 2 neurologic complaints, 2 cognitive features, 1 fever, 1 Sicca
syndrome, 7 skin manifestations (3 facial nodules), 3 evolvement into autoimmune disease. Similar
symptoms/manifestations were reported for other biomaterials.
Clinical findings
: Of the 7 cases who received
HA alone: 1 had livedo reticular and systemic lupus erythematosus. 1 had panniculitis, undifferentiated
connective tissue disease, multiple sclerosis-like. 1 had panniculitis, angioedema, and autoimmune
thyroiditis. 1 had cutaneous vasculitis and autoimmune thyroiditis. 1 had angioedema, panniculitis, facial
nodules, Chron’s disease. 1 had angioedema, facial nodules, myositis, skin induration. 1 had angioedema,
facial nodules, skin induration
Timing of adverse effects: 6 to 317 months following exposure to biomaterial (mean 70.7 months)
Factors that predict response: NR
Source Citation: Alijotas-Reig et al. 2012
73
Study Design: Retrospective single-arm study
Device or Material: Biomaterials injections including HA, acrylamides, or methacrylate compounds
Contact Duration: Onset 12 months or longer after bioimplant injection
Dose: NA
Frequency/Duration: Single administration
Response: Local and Systemic reactions (possible ASIA-related disorders) following injection of different fillers
Patient characteristics (gender, mean age): 15 patients; 14 females/1 male with late-onset inflammatory adverse
reactions related to fillers other than silicone that could be totally or partially considered as ASIA-related
disorders.
Number per group: 15
Observed adverse effects: Local and Systemic Complaints by filler:
Polyalkymide (6/15),
Local: Thigh & buttock
panniculitis, facial nodules, skin indurations; Systemic: Monoclonal gammopathy, fatigue, arthralgia, livedo
reticularis in legs, arthritis, malaise; pain in jaw, primary biliary cirrhosis, sicca syndrome, hands erythema
(vasculitis-like), multiple buttock nodules, feverish, myalgia, rash (vasculitis-like), angioedema,
lymphadenopathy, pneumonitis;
Poly-L-lactic acid (1/15),
Local: facial nodules; Systemic: Malaise, feverish,
cutaneous sarcoidosis
HA/methacrylate (2/15
), Local: facial nodules, indurations; Systemic: Skin rash,
liquen, sicca syndrome, cutaneous sarcoidosis
Polyacrylamide
(1/15
), Local: extensive facial nodules;
Systemic: Malaise, lymphadenopathy, arthritis, rash,
HA (3/15),
Local: None; Systemic: Generalized
cutaneous vasculitis, arthritis, fever, lymphadenopathy, anti-neutrophil citoplasmic antibodies (ANCA),
recurrent angioedema, skin rash, Sjogren’s syndrome,
Unknown filler (1/15)
, Local: nodules in low
back/buttocks; Systemic: Muscle pain and weakness, fatigue, inflammatory polyradiculopathy,
Acrylamide
(1/15),
Local: None; Systemic: sicca syndrome, morphea, polyarthritis

Material Performance Study - Hyaluronic Acid
|
115
Timing of adverse effects: The mean latency between filler or most recent filler injection and appearance of
symptoms was 15.9 months (range one to 48 months).
Factors that predict response: Possible conditions or triggering events: Seven out of the 15 patients had
previously undergone injected procedures using other biomaterials. Two out of 15 patients had had silicone
breast enlargement. In all but four cases, inflammatory signs at the implantation site preceded the distant
or systemic manifestations. Only two patients experienced infectious history as a possible triggering event,
but in both cases the infections were not directly associated with the point of bioimplant injection.
AE = adverse event; AF = autologous fat; AFKG = autologous fibroblast and keratin gel; ASIA = autoimmune/inflammatory
syndrome induced by adjuvants; CaHa = calcium hydroxyapatite; CF = cosmetic filler; CNS = central nervous system; CTR =
common treatment reaction; DAE = delayed adverse event; DTH = delayed-type hypersensitivity; HA = hyaluronic acid; h/o =
history of; ISR = injection site reaction; NR = not reported; SD = standard deviation; TEAE = treatment emergent adverse
events; TRAE = treatment-related adverse events

Material Performance Study - Hyaluronic Acid
|
116
Table 21: Dermal Fillers - Health Effects (In Vivo) Animal Studies
Local Response/ Toxicity
Source Citation: Nie et al. 2019
74
Study Design: Comparative animal study
Device or Material: HA (Restylane) vs PMMA (Artecoll)
Route: Ear (intra-arterial injection)
Dose: 0.1 mL or 0.2 mL
Frequency/Duration: Single administration
Response: Embolism, Necrosis
Species (strain): Rabbit (white)
Gender: Male
Number per group: 30 rabbits total (n = 20 ears for 0.1 ml PMMA and 0.1 ml HA groups; and n = 10 ears for 0.2 ml
PMMA and 0.2 ml HA groups).
Observations on adverse effects (brief): With 0.1 ml injected volume, PMMA was dispersed within a few minutes and
only 5% of the injected ears had mild necrosis on day 7, while HA tended to form obvious transparent
emboli, an indication of blood vessel clotting, and 60% of injected ears showed necrosis on day 7. With 0.2
ml injected volume, PMMA had a risk of complete blood vessel clotting in between 0.1 ml PMMA group and
0.1 ml HA group, and 30% of injected ears had necrosis; in contrast, 100% of 0.2 ml HA-injected ears
showed transparent emboli and necrosis. The necrosis areas were significantly increased in the HA groups
compared with PMMA groups at the same injection volumes. HA injection also caused dilation of small blood
vessels.
Timing of adverse effects: Within 7 days of injection
Factors that predict response: NR
HA: hyaluronic acid; PMMA: poly (methyl methacrylate)

Material Performance Study - Hyaluronic Acid
|
117
Table 22: Intraocular/Ophthalmic Viscoelastic Solution/Fluid - Health Effect (In Vivo) Human Studies
Local Response/ Toxicity
Source Citation: Malvankar-Mehta et al. 2020
75
Study Design: Systematic review
Device or Material: Ophthalmic viscoelastic devices (Healon, Healon5, Healon GV, 2% HPMC, Viscoat, OcuCoat,
Provisc, Soft Shell)
Contact Duration: 1 day to 3 months
Dose: NR
Frequency/Duration: 1 injection/eye
Response: Corneal edema, Intraocular pressure (IOP), Macular edema
Patient characteristics (gender, mean age): Gender NR; mean age ranged from 63 to 77 years across studies.
Number per group: 3893 subjects in 36 RCTs identified were included for analysis.
Observed adverse effects: Significant increase in postoperative IOP in 1-day follow-up with Healon (SMD = 0.37, CI:
[0.07, 0.67]), Viscoat (SMD = 0.29, CI: [0.13, 0.45]), Provisc (SMD = 0.46, CI: [0.17, 0.76]), and Soft Shell
(SMD = 0.58, CI: [0.30, 0.86]) was computed. Conversely, results implied a non-significant increase in
postoperative IOP with Healon GV (SMD = 0.07, CI: [−0.28, 0.41]), Healon5 (SMD = 0.15, CI: [−0.33,
0.64]), 2% HPMC (SMD = 0.32, CI: [−0.0, 0.64]), and OcuCoat (SMD = 0.26, CI: [−0.37, 0.9]).
Additionally, a non-significant reduction in postoperative IOP was inferred with Viscoat + Provisc (SMD =
−0.28, CI: [−2.23, 1.68]). Meta-analysis showed statistically non-significant reduction in post-operative IOP
in 1-week follow-up with Healon (SMD = −0.35, CI: [−0.71, 0.0]), Viscoat (SMD = −0.13, CI: [−0.4, 0.14]),
2% HPMC (SMD = 0.06, CI: [−0.3, 0.42]), and OcuCoat (SMD = −0.41, CI: [−0.98, 0.17]) compared to a
statistically significant reduction in postoperative IOP in 1-week follow-up with Healon GV (SMD = −0.5, CI:
[−0.89, −0.11]) and Healon5 (SMD = −0.42, CI: [−0.68, −0.17]). Statistically significant reductions in IOP
across devices were seen at 1 month, 3 months and 6 months follow-up. Across 4 studies, corneal edema
rates ranged from 0.6% to 2.5%. Across 2 studies, macular edema rates ranged from 0.6% to 0.9%.
Timing of adverse effects: Most events occurred between 1 hour and 1 week post-surgery.
Factors that predict response: NR
Source Citation: Dugan et al. 2020
78
Study Design: Retrospective comparative study
Device or Material: Ophthalmic viscoelastic device (Provisc)
Contact Duration: 3 months
Dose: NR
Frequency/Duration: Single injection
Response: IOP, Microhyphema, Layered hyphema, Choroidal effusions, Tube leak, Other complications
Patient characteristics (gender, mean age): 55% female, mean age 76.4 years
Number per group: Ahmed Glaucoma valve implantation with OVD fill (n = 105) or without OVD fill (n = 54).
Observed adverse effects: IOP did not differ between the 2 groups at any visit, except at 6 months when the OVD-fill
group had lower IOP than the control group (13.8 ± 3.7 vs. 16.6 ± 5.3 mm Hg, P=0.002). At most recent

Material Performance Study - Hyaluronic Acid
|
118
follow-up, the IOP was 15.4 ± 6.4 mm Hg in the OVD-fill group and 15.6 ± 6.9 mm Hg in the non–OVD-fill
group (P=0.47). Compared with baseline, the mean change in IOP was greater in the OVD-fill group at 1-
month follow-up (−17.5 ± 10.2 vs. −13.9± 9.39 mm Hg, P=0.04), but there were no statistically significant
differences at any other postoperative visits (P ≥ 0.06). There were 5 (5.9%) patients in the OVD-fill group
and 3 (8.1%) in the non–OVD-fill group with IOP spikes to ≥30 mm Hg within the first postoperative month
(P=0.70). In the first postoperative month, there were 19 (21.8%) patients with hypotony in the OVD-fill
group and 5 (13.2%) patients in the non–OVD-fill group (P=0.26). There was no difference in complication
rates between the 2 groups, except at week 1 in which 20 of 95 (21.1%) of patients with OVD fill had a
layered hyphema compared with 2 of 45 (4.4%) in the control group (P=0.01). There was 1 patient (1.0%)
in the OVD group and 1 patient (2.2%) in the non-OVD group that required drainage of choroidal effusions
within the first postoperative month. Other complications included a retinal detachment in a patient without
OVD fill and a clogged tube shunt that occurred in a patient who received OVD fill.
Timing of adverse effects: 1 day to 3 months post-surgery.
Factors that predict response: NR
Source Citation: Fezza 2018
81
Study Design: Prospective single-arm study
Device or Material: Cross-linked hyaluronic acid (xlHA) gel occlusive device (Restylane-L™, Q Med, Uppsala, Sweden)
Contact Duration: 6 months
Dose: 0.2 mL
Frequency/Duration: Single injection
Response: No events related to the device
Patient characteristics (gender, mean age): 48 female, 15 male; mean age 67 years
Number per group: 74 patients with dry eye (63 completed the study).
Observed adverse effects: Adverse events were rare and included two cases of periocular itching, which was
attributed to seasonal allergies and one case of conjunctivitis that occurred at month 2 and resolved and
was likely to be an incidental viral infection not related to the gel, as a family member had previously
contracted a “pink eye.” Importantly, there were no cases of dacryocystitis, canaliculitis, swelling, pain,
bruising, or Tyndall effect.
Timing of adverse effects: NR
Factors that predict response: NR
Source Citation: Auffarth et al. 2017
76
Study Design: RCT
Device or Material: Ophthalmic viscoelastic device (Twinvisc and Duovisc)
Contact Duration: 3 months
Dose: Twinvisc (0.7 mL dispersive, 0.7 mL cohesive); Duovisc (0.35 or 0.5 mL dispersive, 0.4 or 0.55 mL cohesive)
Frequency/Duration: Administration of dispersive and cohesive OVD into a single eye
Response: IOP spikes, Inflammation, Ocular hypertension, Corneal edema, Capsule break, Viscoat bubbles
Patient characteristics (gender, mean age): 62% female, mean age 72 years. All patients received cataract surgery.

Material Performance Study - Hyaluronic Acid
|
119
Number per group: 109 (Twinvisc, group 1) and 111 (Duovisc, group 2)
Observed adverse effects: Six hours after the surgery, 7 patients in Group 1 and 8 patients in Group 2 had an IOP of
30 mm Hg or higher. No statistically significant difference between groups at any follow-up was found in
terms of incidence of IOP peaks of 30 mm Hg or higher or 24 mm Hg or higher. All the cases of IOP peaks
of 30 mm Hg or higher resolved either with appropriate medication or paracentesis reopening (6 and 5 eyes
in Group 1 and Group 2, respectively) or spontaneously (1 eye and 3 eyes in Group 1 and Group 2,
respectively). There was evidence of mild inflammation in both groups at 6 hours, 24 hours, and 7 days
postoperatively for all criteria (corneal opacity, inflammatory cells, fibrin, flare, and iritis symptoms).
However, at 30 days and 90 days postoperatively, the inflammation level was very close to baseline values.
There was no statistically significant difference between the 2 groups at any follow-up for any of the criteria,
with the exception of the number of inflammatory cells at 90 days in favor of the OVD 1 group (P = 0.029).
No serious adverse events related to the studied OVDs were reported. Other adverse events potentially
related to the OVDs: In Group 1, there were 14 cases (12.6%) of ocular hypertension, 1 case (0.9%) of
corneal edema, and 1 case (0.9%) of cystoid macular edema. Adverse events in group 2 included 20 cases
(17.6%) of ocular hypertension, 1 case (0.9%) of corneal edema, 1 case (0.9%) of inflammation, 1 case
(0.9%) of capsule break, and 1 report (0.9%) of bubbles in the Viscoat OVD. None of the between-group
differences were statistically significant.
Timing of adverse effects: 6 hours to 3 months post-surgery.
Factors that predict response: NR
Source Citation: Altintas et al. 2017
82
Study Design: Retrospective single-arm study
Device or Material: Ophthalmic viscoelastic device (either 2.0% or 1.4% HA)
Contact Duration: 2 weeks
Dose: NR
Frequency/Duration: Single injection
Response: Toxic anterior segment syndrome (TASS)
Patient characteristics (gender, mean age): 47% female, mean age 67 years
Number per group: 34 patients who developed TASS after routine cataract surgery.
Observed adverse effects: Nine patients developed a moderate degree of corneal edema, mild inflammation of AC in
24–48 h following surgery. No patient had purulent secretion, chemosis, lid involvement, and pain. Other
ten patients underwent phacosurgery at the following day, all of them had corneal edema and AC
inflammation. Four cases out of ten had severe corneal stromal edema extending from limbus to limbus
combining with fibrin formation in the AC, and two cases had hypopyon, but conjunctival inflammation was
minimal without discharge and chemosis. None of them had periocular pain. The authors identified the
source of TASS as OVD derived from rooster comb; when they substituted with OVD derived from bacterial
fermentation no further cases of TASS were observed.
Timing of adverse effects: Initial symptoms occurred within 1 day following surgery.
Factors that predict response: OVD derived from rooster comb that had been stored improperly was responsible for
the outbreak.
Source Citation: Jaramillo et al. 2014
83
Study Design: Retrospective single-arm study

Material Performance Study - Hyaluronic Acid
|
120
Device or Material: Ophthalmic viscoelastic device (Healon GV, 1.4% HA)
Contact Duration: 12 months
Dose: NR
Frequency/Duration: Healon GV was injected every 2 to 3 clock hours to a total of 8 times in all cases.
Response: Descemet membrane detachment (DMD) with or without intracorneal hemorrhage
Patient characteristics (gender, mean age): The average age of the DMD group was 68. 6 years, and there were 7
male and 5 female patients. Mean age for all patients was 68.5 years.
Number per group: 162 eyes of 115 patients who received canaloplasty for glaucoma.
Observed adverse effects: Twelve patients developed DMD after canaloplasty (7.4%) (12 eyes of 162). Intracorneal
hemorrhage within DMD occurred in 58% (7/12), whereas 42% (5/12) developed DMD with intracorneal
viscoelastic (Healon GV) alone. Two patients had large detachments measuring 5 to 6 mm extending into
the visual axis. DMD resolved completely with or without drainage except for 1 patient who developed
corneal decompensation, needing penetrating keratoplasty.
Timing of adverse effects: DMD was noticed at the time of surgery in all cases. All cases resolved in 4 to 10 weeks (3
patients required additional surgery to resolve the DMD).
Factors that predict response: No between-group differences in baseline variables were identified between DMD and
non-DMD eyes. The authors noted “DMD could be related to excessive amounts of Healon GV injection into
the Schlemm canal during the viscodilation portion of the surgery.”
Source Citation: Uemoto et al. 2013
79
Study Design: Retrospective comparative study
Device or Material: Ophthalmic viscoelastic device with brilliant blue G (Visco-BBG) versus balanced salt solution with
BBG (BSS-BBG)
Contact Duration: Visco-BBG was removed 30 seconds after injection
Dose: 0.3 mL
Frequency/Duration: Single injection
Response: No complications with Visco-BBG
Patient characteristics (gender, mean age): All patients had undergone par plana vitrectomy (PPV) combined with
internal limiting membrane (ILM) peeling. Patient characteristics NR.
Number per group: Visco-BBG (40 eyes), BSS-BBG (34 eyes)
Observed adverse effects: No ocular hypertension or undue inflammation (endophthalmitis) attributable to residual
visco-BBG was evident postoperatively. There were no alterations of the retinal pigment epithelium due to
dye toxicity in all cases. An accidental retinal perforation occurred in an epimacular membrane case of the
BSS-BBG group.
Timing of adverse effects: The only adverse event occurred during surgery.
Factors that predict response: NR
Source Citation: Leszczyski et al. 2012
80
Study Design: Prospective comparative study
Device or Material: Ophthalmic viscoelastic device (SKGEL, reticulated HA)
Material Performance Study - Hyaluronic Acid
|
121
Contact Duration: Follow-up was 24 months
Dose: NR
Frequency/Duration: Single injection
Response: See intraoperative, early post-operative and late post-operative complications under observed adverse
events
Patient characteristics (gender, mean age): 28 men and 40 women, mean age 64.8±11.6 years. The study compared
non-penetrating very deep sclerectomy (NPVDS) with the use of hyaluronic acid implant (SKGEL) to
trabeculectomy (TB) in patients with medically uncontrolled glaucoma.
Number per group: NPVDS (39 eyes); TB (39 eyes)
Observed adverse effects:
Intraoperative: NPVDS TB p-value
Anterior chamber flattening 1 (2.6 %) 4 (10.3 %) 0.029
Anterior chamber hemorrhage 1 (2.6 %) 4 (10.3 %) 0.029
Perforation of
trabeculo-Descemet membrane 2 (5.1 %) 0 (0.0 %) 0.0676
Early post-operative:
Choroidal detachment 2 (5.1 %) 4 (10.3 %) 0.124
Hyphema 2 (5.1 %) 5 (12.8 %) 0.054
Inflammation 0 (0.0 %) 1 (2.6 %) 0.079
Late post-operative:
Cataract progression 4 (10.3 %) 12 (30.8 %) 0.002
Bleb fibrosis 2 (5.1 %) 1 (2.6 %) 0.207
All complications: 14 (35.9 %) 31 (79.5 %) 0.00011
Overall complication rate higher in TB group.
Timing of adverse effects: Intraoperative to late post-operative, but timing of late events not reported.
Factors that predict response: NR
Source Citation: Modi et al. 2011
77
Study Design: RCT
Device or Material: Ophthalmic viscoelastic device (DisCoVisc or Healon)
Contact Duration: 3 months
Dose: 0.85 mL (Healon), 1 mL (DisCoVisc)
Frequency/Duration: Single injection (Healon), double injection (DisCoVisc)
Response: IOP ≥30mmHg, Aqueous cells, Severe corneal edema, Ocular discomfort, Conjunctival hyperemia,
Conjunctival injection, Conjunctival erythema
Patient characteristics (gender, mean age): 63% female, mean age 69 to 70 years between groups.
Number per group: DisCoVisc (128 eyes), Healon (121 eyes)

Material Performance Study - Hyaluronic Acid
|
122
Observed adverse effects: At 6 hours after surgery, both OVD groups had 15 patients with IOP ≥ 30 mmHg, yielding
a similar percentage of patients with IOP ≥ 30 mmHg (13.0% of DisCoVisc OVD patients, 13.3% of Healon
OVD patients). At 24 hours after surgery, both OVD groups had seven patients with IOP ≥ 30 mmHg
(similar between groups, at 6.1% of DisCoVisc OVD patients and 6.2% of Healon OVD patients). By
postoperative day 7, only one Healon OVD patient (0.9% of cases) and no DisCoVisc OVD patients had IOP
≥ 30 mmHg. No IOPs ≥ 30 mmHg were observed in any patient following postoperative day 13. For the
aqueous cells parameter, 3 eyes exhibited clinically significant scores: two eyes in the Healon OVD group
(one eye at 1 day postoperative and one eye at 7 days postoperative) and one eye in the DisCoVisc OVD
group at an unscheduled visit (41 days postoperative). The latter case was not related to the OVD, but was
attributed to residual lens fragments after surgery in an eye with a very small pupil, and was resolved after
treatment with prednisolone acetate and atropine. Severe corneal edema was observed in one eye in the
DisCoVisc OVD group and in one eye in the Healon OVD group; both cases occurred 1 day postoperative.
The most frequently reported clinical observations of events related to the safety and tolerability of the
OVDs occurred in both study groups and included ocular discomfort, conjunctival hyperemia, conjunctival
injection, or conjunctival erythema.
Timing of adverse effects: See above
Factors that predict response: NR
Source Citation: Shafi et al. 2011
84
Study Design: Retrospective single-arm study
Device or Material: Ophthalmic viscoelastic device (Healon GV, 1.4% HA)
Contact Duration: Follow-up 12 months
Dose: 0.4 mL of Healon GV plus subconjunctival injection of 5-FU (10 mg in 0.4 mL)
Frequency/Duration: Single injection of Healon GV, single injection of 5-FU
Response: Reported complications likely related to the needling procedure, not Healon GV
Patient characteristics (gender, mean age): 41.5% female, mean age 67.13 ± 15.76 years
Number per group: 46 patients (53 eyes) who had undergone primary bleb needling revision (BNR) with adjunctive
5-FU and routine subconjunctival Healon GV.
Observed adverse effects: Of 53 needling procedures, 16 eyes (30%) had a complication. Reported complications
(early hypotony, needle-track leak, subconjunctival hemorrhage, anterior uveitis flareup) more likely related
to the needling procedure than Healon GV, a controlled study would be needed to determine complications
related to Healon GV.
Timing of adverse effects: Most complications occurred and were resolved within 2 weeks post-needling.
Factors that predict response: NR
FU = fluorouracil; HA = hyaluronic acid; IOP = intraocular pressure; NA = not applicable; NR = not reported; OVD =
ophthalmic viscoelastic device; RCT = randomized controlled trial

Material Performance Study - Hyaluronic Acid
|
123
Table 23: Eye Drops - Health Effect (In Vivo) Human Studies
Local Response/ Toxicity
Source Citation: Groβ et al. 2018
85
Study Design: RCT
Device or Material: Hyaluronic acid (HA) drops versus 0.5% carboxymethylcellulose (CMC) drops for keratitis or
keratoconjunctivitis related to dry eye disease
Contact Duration: NR
Dose: 1 drop
Frequency/Duration: 3 times/day for 35 days
Response: Chronic arthrosis, Episodic crackling feeling under the lid, Episodic chalazion, Chronic eye stinging
Patient characteristics (gender, mean age): Female = 56 (70%), Male = 24 (30%); mean age = 55.81 years
Number per group: HA = 41, CMC = 39
Observed adverse effects: 4 adverse events (9.7%) in HA group (chronic arthrosis, episodic crackling feeling under
the lid, episodic chalazion, chronic eye stinging) 3 adverse events (5.1%) in the CMC group (episodic lid
edema, episodic eye in one patient stinging, chronic lid eczema in one patient) Stinging was improved more
with HA compared to CMC on day 84 (p=0.0106) Itching was improved more with HA compared to CMC on
day 84 (p=0.0179) No difference in pain was observed between the two groups
Timing of adverse effects: Within 85 days
Factors that predict response: NR
Source Citation: Labetoulle et al. 2017
93
Study Design: RCT
Device or Material: Osmoprotectants, carboxymethylcellulose and hyaluronic acid (O/CMC/HA) versus HA drops for
moderate to severe symptomatic dry eye
Contact Duration: NR
Dose: 1-2 drops
Frequency/Duration: 2-6 times/day for 3 months
Response: Eye pruritus, dry eye, eye irritation, eyelid irritation
Patient characteristics (gender, mean age): Female = 100%; mean age = 62.9 (O/CMC/HA) & 61.4 (HA)
Number per group: 40
Observed adverse effects: Eye pruritus (n=2, 5%) was reported in the O/CMC/HA group, Dry eye (n=2, 5%) was
reported in the HA group, Eye irritation (n=2, 5%), was reported in the HA group, Eyelid irritation (n=1,
2.5%) was reported in the HA group. In the O/CMC/HA group, 9 (22.5%) adverse events were mild, 4
(10%) adverse events were moderate and 3 (7.5%) adverse events were severe. In the HA group, 11
(27.5%) adverse events were mild, 4 (10%) adverse events were moderate and 1 (2.5%) adverse event
was severe.
Timing of adverse effects: Within 3 months
Factors that predict response: NR

Material Performance Study - Hyaluronic Acid
|
124
Source Citation: Cagini et al. 2017
86
Study Design: RCT
Device or Material: Cross linked HA (0.2%) versus HA (0.2%) for Sjögren syndrome-related dry eye
Contact Duration: 60 minutes
Dose: 1 drop
Frequency/Duration: Once
Response: None
Patient characteristics (gender, mean age): 100% Female (20); mean age = 57.8 years
Number per group: Treatment group = 20, control group = 20
Observed adverse effects: No stinging or other adverse events in either group
Timing of adverse effects: 60 minutes
Factors that predict response: NR
Source Citation: Groβ et al. 2017
87
Study Design: RCT
Device or Material: 0.2% HA (Hylo Confort Plus/HydoGel) versus 0.18% HA (Vismed® Multi) for moderate to severe
dry eye and noninfectious, nonviral keratitis, or keratoconjunctivitis
Contact Duration: 35 days
Dose: NR
Frequency/Duration: NR
Response: Grittiness exacerbation, Important ocular fatigue + ocular irritation, Ocular irritation
Patient characteristics (gender, mean age): 59 Female (69.4%), 26 male (30.6%); Mean age = 59.7 years
Number per group:
0.2% HA = 38; 0.18% HA = 32
Observed adverse effects: The rate of adverse events (AE) was 2.3% for 0.2% HA (1 episodic allergy) and 7.1% for
0.18% HA (1 grittiness exacerbation, 1 ocular irritation, 1 important ocular fatigue + ocular irritation) with
no serious AE.
Timing of adverse effects: 84 days
Factors that predict response: NR
Source Citation: Lambiase et al. 2017
88
Study Design: RCT
Device or Material: Lubricin versus HA for grade 2-3 moderate dry eye disease
Contact Duration: 14 days
Dose: 2-6 drops
Frequency/Duration: Once/day for 7 days

Material Performance Study - Hyaluronic Acid
|
125
Response: Transient adverse events
Patient characteristics (gender, mean age): Lubricin: 94.7% female, 5.3% male; mean age = 51.9 years. HA: 90%
female, 2% male; mean age 61.8 years.
Number per group: Lubricin: N=19. HA: N=20.
Observed adverse effects: Transient adverse events: lubricin=2, HA=2, Treatment related adverse events: none
Timing of adverse effects: 14 days
Factors that predict response: NR
Source Citation: Chiambaretta et al. 2017
94
Study Design: RCT
Device or Material: HA-trehalose (Thealoz Duo®/Thealose®) versus HA (Vismed®) for moderate to severe dry eye
disease
Contact Duration: 84 days
Dose: 1 drop
Frequency/Duration: 3-6 times/day for 84 days
Response: Eye disorders, eye pruritus, chalazion, conjunctival irritation, conjunctivitis allergic, eye irritation, eye pain,
keratitis, ulcerative keratitis, infections, conjunctivitis viral
Patient characteristics (gender, mean age): HA-trehalose: 11 (21.2%) male, 41 (78.8%) female, mean age = 60
years. HA: 8 (15.1%) male, 45 (84.9%) female, mean age = 58.5 years. Overall mean age = 59.2 years
Number per group: HA-trehalose = 52; HA=53
Observed adverse effects: At least one ocular treatment-emergent AE: HA-trehalose = 1 (1.9%), HA = 7 (13.2%)
Eye disorders: HA trehalose = 0, HA = 6, Eye pruritus: HA trehalose = 0, HA = 2, Chalazion: HA trehalose =
0, HA = 1, Conjunctival irritation: HA trehalose = 0, HA = 1, Conjunctivitis allergic: HA trehalose = 0, HA =
1, Eye irritation: HA trehalose = 0, HA = 1, Eye pain: HA trehalose = 0, HA = 1, Keratitis: HA trehalose = 0,
HA = 1, Ulcerative keratitis: HA trehalose = 0, HA = 1, Infections and infestations: HA trehalose = 1, HA =
1, Conjunctivitis viral: HA trehalose = 1, HA = 1, Significantly fewer adverse events in the HA-trehalose
group, compared to the HA group.
Timing of adverse effects: 84 days
Factors that predict response: NR
Source Citation: Robert et al. 2016
95
Study Design: RCT
Device or Material: Cationic emulsion (CE) versus 0.18% hyaluronate sodium (HS) for moderate to severe dry eye
disease.
Contact Duration: 3 months
Dose: 1 drop
Frequency/Duration: 4 times/day
Response: Installation site erythema, instillation site pain, instillation site pruritus, blepharitis, conjunctival
hyperemia, conjunctivitis, eye irritation, eye pain
Material Performance Study - Hyaluronic Acid
|
126
Patient characteristics (gender, mean age): 9 (20.5%) male, 35 (79.5% female in the CE group. 7 (17.1%) male, 34
(82.9%) female in the HS group. 16 (18.8%) male, 69 (81.2%) female overall. Mean age = 60 years in the
CE group. Mean age = 65.3 years in the HA group. Mean age = 62.6 years overall.
Number per group: CE = 44. HS = 41.
Observed adverse effects: Patients with at least one adverse event: CE = 8, HS = 11. Patients with at least one
ocular adverse event: CE = 7, HS = 8. Patients with treatment-related adverse event: CE = 3, HS = 4.
Installation site erythema: CE = 1, HS = 0. Instillation site pain: CE = 2. HS = 1. Instillation site pruritus: CE
= 0, HS = 1. Blepharitis: CE = 1, HS = 0. Conjunctival hyperemia: CE = 0, HS = 1. Conjunctivitis: CE = 1,
HS = 0. Eye irritation: CE = 0, HS = 1. Eye pain: CE = 0, HS = 1. No major differences between treatments
were found with respect to local ocular tolerance.
Timing of adverse effects: 3 months
Factors that predict response: NR
Source Citation: Gong et al. 2015
89
Study Design: RCT
Device or Material: Diquafosol ophthalmic solution (diquafosol) versus sodium hyaluronate solution (HA) for dry eye
Contact Duration: 6 weeks
Dose: NR
Frequency/Duration: 6 times/day
Response: Mild and moderate treatment related adverse events and eye disorders
Patient characteristics (gender, mean age): Diquafosol: 53 (22%) male, 188 (78%) female HA: 55 (22.2%) male,
193 (77.8%) female. Mean age not reported.
Number per group: Diquafosol = 241, HA = 248
Observed adverse effects: Mild adverse events: diquafosol = 17, HA = 11, Moderate adverse events: diquafosol =
13, HA = 4, No severe adverse events. There are statistically significantly less adverse events in the HA
group compared to the diquafosol group (p=0.0186). Mild eye disorders: diquafosol = 17, HA = 11.
Moderate eye disorders: diquafosol = 12, HA = 4. No severe eye disorders.
There are statistically significantly less eye disorders in the HA group compared to the diquafosol group (p=0.0267).
Timing of adverse effects: NR
Factors that predict response: NR
Source Citation: Prabhasawat et al. 2015
90
Study Design: RCT
Device or Material: 0.3% Hydroxypropyl methylcellulose/dextran (TearsNaturale®) versus 0.18% sodium hyaluronate
(Vislub®) for moderate to severe dry eye disease
Contact Duration: 28 days
Dose: 1 drop
Frequency/Duration: 4 times/day
Response: none

Material Performance Study - Hyaluronic Acid
|
127
Patient characteristics (gender, mean age): HPMC/dextran: 10 (28.6%) male, 25 (71.4%) female. SH: 13 (37.1%)
male, 22 (62.9%) female
Number per group: HPMC/dextran = 35 SH = 35
Observed adverse effects: No serious adverse events were reported for either group
Timing of adverse effects: 28 days
Factors that predict response: N/A
Source Citation: Jee et al. 2014
91
Study Design: RCT
Device or Material: Preservative free 0.1% fluorometholone (Humeron) and 0.1% sodium hyaluronate (Tearin) (1
month) then 0.1% sodium hyaluronate and 0.05% cyclosporine (Restasis) (2 months) versus preserved
0.1% fluorometholone (Ocumetholone) and 0.1% sodium hyaluronate (Lacure) (1 month) then 0.1%
sodium hyaluronate and 0.05% cyclosporine (Restasis) (2 months) for moderate to severe dry eye
syndrome.
Contact Duration: 3 months
Dose: NR
Frequency/Duration: 2-4 times/day
Response: Stinging eyes
Patient characteristics (gender, mean age): Preservative free: 15 male, 35 female; mean age NR. Preserved: 12
male, 38 female; mean age NR
Number per group: 50
Observed adverse effects: Preservative free: 3 (6%), Preserved: 5 (10%)
Timing of adverse effects: 3 months
Factors that predict response: NR
Source Citation: Kinoshita et al. 2013
96
Study Design: RCT
Device or Material: 2% rebamipide versus 0.1% sodium hyaluronate for dry eye
Contact Duration: 4 weeks
Dose: 1 drop
Frequency/Duration: 4-6 times/day
Response: Conjunctival hemorrhage, eye irritation, eye pain, visual impairment, eye pruritus
Patient characteristics (gender, mean age): Rebamipide: 10 (10.8%) male, 83 (89.2%) female; mean age NR.
Sodium Hyaluronate: 15 (15.8%) male, 80 (84.2%) female; mean age NR
Number per group: Rebamipide = 93 Sodium, Hyaluronate = 95
Observed adverse effects: Conjunctival hemorrhage: Rebamipide = 1 (1.1%), Sodium hyaluronate = 2 (2.1%), Eye
irritation: Rebamipide =0, Sodium hyaluronate = 2 (2.1%), Eye pain: Rebamipide = 0, Sodium hyaluronate

Material Performance Study - Hyaluronic Acid
|
128
= 3 (3.2%), Visual impairment: Rebamipide = 2 (2.2%), Sodium hyaluronate = 0, Eye pruritus: Rebamipide
= 4 (4.3%), Sodium hyaluronate = 2 (2.1%)
Timing of adverse effects: 4 weeks
Factors that predict response: NR
Source Citation: Liu et al. 2012
92
Study Design: RCT
Device or Material: 0.1% topical pranoprofen + 0.1% topical sodium hyaluronate versus 0.1% sodium hyaluronate
for dry eye
Contact Duration: 30 days
Dose: NR
Frequency/Duration: 4 times/day
Response: None
Patient characteristics (gender, mean age): Prenoprofen/sodium hyaluronate: 18 female, 12 male; mean age = 41.51
years. Sodium hyaluronate: 20 female, 10 make; mean age = 42.12 years
Number per group: 30
Observed adverse effects: None observed
Timing of adverse effects: 30 days
Factors that predict response: NR
Source Citation: Takamura et al. 2012
97
Study Design: RCT
Device or Material: 3% diquafosol versus 0.1% sodium hyaluronate for dry eyes
Contact Duration: 2 weeks
Dose: 1 drop
Frequency/Duration: 6 times/day
Response: Blepharitis, eye discharge, eye irritation, eye pain, foreign body sensation, conjunctival hyperaemia, eye
pruritus, ocular discomfort
Patient characteristics (gender, mean age): Diquafosol: 120 (83.3%) female; mean age = 55.3 years. Sodium
hyaluronate: 125 (88%) female; mean age = 56.9 years
Number per group: Diquafosol = 144. Sodium hyaluronate = 139
Observed adverse effects: No statistically significant difference in adverse events between groups. Adverse drug
reaction incidence rates were significantly lower in the sodium hyaluronate group (4.9%) compared to the
diquafosol group (15.3%), p=0.005. Blepharitis: Diquafosol = 0, Sodium hyaluronate = 2 (1.4%). Eye
discharge: Diquafosol = 4 (2.8%), Sodium hyaluronate = 0. Eye irritation: Diquafosol = 9 (6.3%), Sodium
hyaluronate = 1 (0.7%). Eye pain: Diquafosol = 2 (1.4%), Sodium hyaluronate = 1 (0.7%). Foreign body
sensation: Diquafosol = 4 (2.8%), Sodium hyaluronate = 1 (0.7%). Conjunctival hyperaemia: Diquafosol =
2 (1.4%), Sodium hyaluronate = 1 (0.7%). Eye pruritus: Diquafosol = 2 (1.4%), Sodium hyaluronate = 2

Material Performance Study - Hyaluronic Acid
|
129
(1.4%). Occular discomfort: Diquafosol = 2 (1.4%), Sodium hyaluronate = 0. No serious adverse events in
either group.
Timing of adverse effects: 4 weeks
Factors that predict response: NR
Source Citation: Baudouin et al. 2012
98
Study Design: RCT
Device or Material: OsPR-CMC (OPTIVE®) versus Na-HY (Vismed®) for dry eye
Contact Duration: 3 months
Dose: 1 drop
Frequency/Duration: 3-6 times/day
Response: Conjunctival hemorrhage, Conjunctival hyperemia, Cystitis, Erythema of eyelid, Infection, Installation site
pain, Keratitis, Meibomian gland dysfunction, Nasopharyngitis, Pain, Viral conjunctivitis
Patient characteristics (gender, mean age): OPTIVE®: 35 (87.5%) female; mean age = 58.1 years. Vismed®: 34
(91.9%) female; mean age = 55.4 years
Number per group: OPTIVE® = 40. Vismed® = 37
Observed adverse effects: EYE DISORDERS - Keratitis: OPTIVE® = 1 (2.4%), Vismed® = 1 (2.6%), Conjunctival
hemorrhage: OPTIVE® = 1 (2.4%), Vismed® = 0, Conjunctival hyperemia: OPTIVE® = 0, Vismed® = 1
(2.6%), Erythema of eyelid: OPTIVE® = 0, Vismed® = 1 (2.6%), Meibomian gland dysfunction: OPTIVE®
= 0, Vismed® = 1 (2.6%)
INFECTIONS - Viral conjunctivitis: OPTIVE® = 0, Vismed® = 1 (2.6%), Cystitis: OPTIVE® = 0, Vismed® =
1 (2.6%), Nasopharyngitis: OPTIVE® = 0, Vismed® = 1 (2.6%)
GENERAL - Installation site pain: OPTIVE® = 0, Vismed® = 1 (2.6%)
Timing of adverse effects: 3 months
Factors that predict response: NR
AE = adverse events; CE = cationic emulsion; CMC = carboxymethylcellulose; HA = hyaluronic acid; HPMC = hydroxypropyl
methylcellulose; HS = hyaluronate sodium; NR = not reported; O = osmoprotectants; RCT = randomized controlled trial

Material Performance Study - Hyaluronic Acid
|
130
Appendix D3. Evidence Tables – Adhesion Barrier and Bulking
Agent Applications
Table 24: Hyaluronic acid as a Material – Health Effect (In Vivo) Human Studies
Local Response/ Toxicity
Source Citation: Redorta et al. 2021
101
Study Design: Multicenter, unblinded RCT of men undergoing radiotherapy for prostate cancer
Device or Material: HA and CS instilled into the bladder (intravesical) prior to radiation therapy plus oral intake.
Control group received no treatment.
Contact Duration:
Dose: Sterile solution containing sodium HA 1.6%, 800 mg/50 ml and CS 2%, 1 g/50 ml (HA-SC; iAluRil Prefill; IBSA,
Lodi, Italy) for bladder. Oral: 200 mg of CS, 20 mg of HA, 200 mg of quercetin, and 100 mg of curcumin.
Frequency/Duration: Weekly intravesical instillation for 6 weeks plus oral formulation twice a day for 12 weeks
Response: Haematuria
Patient characteristics (gender, mean age): HA/CS: 67 years, all male. Control: 71 years, all male
Number per group: HA/CS: 25. Control: 24
Observed adverse effects: “Intravesical treatment was very well tolerated, as substantiated by full compliance for all
patients, who retained the intravesical solution in the bladder for at least 30 min without any distress.
Overall, there were seven mild to-moderate (grade 1–2) drug-related adverse events, including
haematuria,
and nausea
, and urticaria reported by three patients. There were no serious (grade 3–4) drug-related
adverse events or treatment-related withdrawals.” No reports of adverse events in the control group.
Timing of adverse effects: ---
Factors that predict response: Lacks a placebo control group.
Systemic Response/ Toxicity
Source Citation: Redorta et al. 2021
101
Study Design: Multicenter, unblinded RCT of men undergoing radiotherapy for prostate cancer
Device or Material: HA and CS instilled into the bladder (intravesical) prior to radiation therapy plus oral intake.
Control group received no treatment.
Contact Duration:
Dose: Sterile solution containing sodium HA 1.6%, 800 mg/50 ml and CS 2%, 1 g/50 ml (HA-SC; iAluRil Prefill; IBSA,
Lodi, Italy) for bladder. Oral: 200 mg of CS, 20 mg of HA, 200 mg of quercetin, and 100 mg of curcumin.
Frequency/Duration: Weekly intravesical instillation for 6 weeks plus oral formulation twice a day for 12 weeks
Response: Nausea, Uticaria
Patient characteristics (gender, mean age): HA/CS: 67 years, all male. Control: 71 years, all male
Number per group: HA/CS: 25. Control: 24

Material Performance Study - Hyaluronic Acid
|
131
Observed adverse effects: “Intravesical treatment was very well tolerated, as substantiated by full compliance for all
patients, who retained the intravesical solution in the bladder for at least 30 min without any distress.
Overall, there were seven mild to-moderate (grade 1–2) drug-related adverse events, including haematuria,
nausea, and
urticaria
reported by three patients. There were no serious (grade 3–4) drug-related adverse
events or treatment-related withdrawals.”
Timing of adverse effects: ---
Factors that predict response: Lacks a placebo control group.
NA = not applicable; NR = not reported; Obs = observational; Retro = retrospective; R = reliable; Dose = mg/kg/day; HA =
hyaluronic acid; CS = chondroitin sulfate

Material Performance Study - Hyaluronic Acid
|
132
Table 25: Adhesion Barrier - Health Effects (In Vivo) Human Studies
Local Response/ Toxicity
Source Citation: Borghese et al. 2021
120
Study Design: SR of absorbable adhesion barriers to prevent adhesion formation after laparoscopic myomectomy
(surgical procedure to remove uterine fibroids)
Device or Material: Auto-cross-linked HA gel and HA+CMC versus control
Contact Duration: NR
Dose: NR
Frequency/Duration: Single administration
Response: None reported
Patient characteristics (gender, mean age): Review with 8 RCTs involving 748 patients. Intervention group: mean
age range 28.8 to 37 years, Control group: mean age range 30.1 to 44.3 years.
Number per group: Intervention group: n=392, Control group: n=356.
Observed adverse effects: “None of the selected studies reported any serious adverse event nor complication related
to anti-adhesion materials application.”
Timing of adverse effects: NR
Factors that predict response: NR
Source Citation: Guo et al. 2021
103
Study Design: SR of Seprafilm on postoperative small bowel obstruction
Device or Material: Seprafilm
Contact Duration: Absorbed
Dose: 1 or more sheets, surface area varied
Frequency/Duration: Single administration
Response: Small bowel obstruction, Anastomotic leaks, Fistulae
Patient characteristics (gender, mean age): 9 clinical control trials involving 4,351 patients were included. 4 RCTs
Number per group: 2,123 in the Seprafilm group and 2,228 in the control group
Observed adverse effects: “The overall analysis showed that the pooled risk ratio was 0.45 (95% confidence interval
= 0.34 to 0.60; P < .00001), indicating that the risk of postoperative small bowel obstruction can be
significantly decreased by the application of Seprafilm.”
Timing of adverse effects: NR
Factors that predict response: “Although Seprafilm is used to decrease adhesions and obstructions, it is also
associated with an increase in anastomotic leaks and fistulae, especially when wrapped around the bowel.”
Source Citation: Hajibandeh et al. 2021
102
Study Design: SR of Seprafilm use on outcomes of abdominal surgery

Material Performance Study - Hyaluronic Acid
|
133
Device or Material: HA+CMC barrier (Seprafilm) versus no treatment (control)
Contact Duration: NR
Dose: NR
Frequency/Duration: Single administration
Response: Anastomotic leak, Ileus, Intra‑abdominal abscess, small bowel obstruction, SSIs
Patient characteristics (gender, mean age): Review of 13 RCTs involving 3,665 patients. Patient characteristics NR
Number per group: Seprafilm group: n= 1800, Control group: n=1865
Observed adverse effects: Use of Seprafilm was associated with significantly higher risk of anastomotic leak (3.1%)
compared to control (1.6%) (RR 1.85, 95% CI 1.15–3.00, p=0.01). The rate of ileus in the Seprafilm group
(4.7%) was not significantly different than the control group (4.8%) (RR 0.97 95% CI 0.68–1.38, p=0.87).
The rate of intra-abdominal abscess in the Seprafilm group (3.6%) was not significantly different than the
control group (2.5%) (RR 1.46, 95 CI 0.92–2.32, p=0.11). Use of Seprafilm was associated with significantly
lower incidence of small bowel obstruction (3.0%) compared to control (5.9%) (RR 0.53, 95% CI 0.38–
0.73, p=0.0001). The SSI rates were not significantly different between the Seprafilm (5.2%) and control
(4.3%) groups (RR 1.21, 95 CI 0.86–1.70, p=0.28).
Timing of adverse effects: NR
Factors that predict response: Use of Seprafilm in lower gastrointestinal surgeries, open surgeries, elective surgeries,
and to treat benign disease demonstrated significantly lower rates of small bowel obstruction and higher
rates of anastomotic leaks.
Source Citation: Tafti et al. 2021
128
Study Design: Single-center, double-blind RCT of females undergoing uterine septum resection
Device or Material: Intrauterine injection of HA
Contact Duration: Absorbed after 7 days
Dose: 1 mL HA gel (100% HA)
Frequency/Duration: Single administration
Response: None reported
Patient characteristics (gender, mean age): 65 females. HA group 34 years, Control group 33.5 years
Number per group: HA group: n=34, Control group: n=31
Observed adverse effects: “Evidenced by the results, intrauterine injection of hyaluronic acid reduced the incidence of
Asherman’s syndrome [IUA] in the case [HA] group than the controls with no reported complications.”
Timing of adverse effects: 2 months
Factors that predict response: NR
Source Citation: Zhao et al. 2021
104
Study Design: SR of Seprafilm use to prevent intestinal obstruction after
gastrointestinal neoplasms surgery
Device or Material: Seprafilm versus control
Contact Duration: NR

Material Performance Study - Hyaluronic Acid
|
134
Dose: 1 or more sheets
Frequency/Duration: Single administration
Response: Intestinal obstruction, Complications, Anastomotic leakage, Intra-abdominal infection, Wound infection
Patient characteristics (gender, mean age): Review of 5 RCTs and 5 clinical studies involving 2,937 patients. Patient
characteristics were not reported equally across the 10 studies.
Number per group: Seprafilm group n=1334, Control group n=1603
Observed adverse effects: The incidence of postoperative intestinal obstruction was significantly lower in the
Seprafilm group than the control (RR, 0.52; 95% CI, 0.38–0.70; p<0.0001). Post-operative complications
were significantly lower in the Seprafilm group compared to control (RR, 0.77; 95% CI, 0.61–0.97; p = .03)
with low heterogeneity (I
2
=24%). Both groups had a similar incidence of: anastomotic leakage (RR, 1.25;
95% CI, 0.68–2.31; p=.48) with low heterogeneity (I
2
=0%), intra-abdominal infection (RR, 0.86; 95% CI,
0.46–1.62; p=.65) with low heterogeneity (I
2
=0%), and postoperative wound infection (RR, 0.85; 95% CI,
0.39–1.85; p=.68).
Timing of adverse effects: NR
Factors that predict response: “The results of the subgroup analyses based on numbers of Seprafilm used showed
that there were significant differences in the incidence of postoperative intestinal obstruction [1 sheet (RR,
0.48; 95% CI, 0.28–0.80); p=.005); 2 sheets (RR, 0.57; 95% CI, 0.34–0.96; p=.04)]”. “We concluded that
there was no difference in the incidence of anastomotic leakage between the Seprafilm group and the
control group. This may be related to the fact that surgeons avoid using Seprafilm around the anastomosis
during surgery”.
Source Citation: Ekin et al. 2020
131
Study Design: Single-center RCT of females who underwent laparoscopic surgery due to Deep Infiltrating
Endometriosis
Device or Material: NCH gel
Contact Duration: Absorbed
Dose: 40 mL of NCH gel
Frequency/Duration: Single administration
Response: Pain, Dysmenorrhoea, Dyschezia (constipation associated with a defective reflex for defecation),
Dyspareunia (genital pain associated with sexual intercourse)
Patient characteristics (gender, mean age): 124 females. NCH gel: 34.4 years, Sterile saline control: 36.4 years
Number per group: NCH gel: n=62, Sterile saline control: n=62
Observed adverse effects: “There was a statistically significant reduction in dysmenorrhoea, dyschezia, dyspareunia
at 3rd and 6th month, and in VAS scores at 6th month in NCH gel group compared to the control group.”
Timing of adverse effects: NR
Factors that predict response: “One of the reasons why NCH gel may have a positive effect on the quality of life
scores can be explained by its anti-proliferative effects on endometriotic implants.”
Source Citation: Fei et al. 2020
124
Study Design: SR of HA gel to prevent IAU formation after miscarriage
Device or Material: HA gel versus control

Material Performance Study - Hyaluronic Acid
|
135
Contact Duration: NR
Dose: NR
Frequency/Duration: Single administration
Response: None reported
Patient characteristics (gender, mean age): Review of 4 RCTs involving 625 females. Patient age NR
Number per group: HA group: n=290, Control group: n=335
Observed adverse effects: No complications were reported for the HA and control groups.
Timing of adverse effects: 6 weeks to 8 months
Factors that predict response: “The matrix composed of hyaluronic acid and fibrin is gradually degraded, and low-
molecular-weight hyaluronic acid produced by degradation promotes angiogenesis, which plays an
important role in wound healing and helps to prevent the occurrence of adhesions.”
Source Citation: Mao et al. 2020
114
Study Design: Single-center RCT of females with moderate to severe IUAs
Device or Material: NCH gel (MateRegen)
Contact Duration: NR
Dose: NR
Frequency/Duration: Single administration
Response: None reported
Patient characteristics (gender, mean age): 306 females. MateRegen group mean age 35.9 years. Control group
mean age 36.7 years.
Number per group: MateRegen group: n=202, Control group: n=104
Observed adverse effects: “No adverse reaction was observed in both groups of participants.”
Timing of adverse effects: NR
Factors that predict response: NR
Source Citation: Wang et al. 2020
133
Study Design: Single-center RCT of females after operative hysteroscopy for intrauterine adhesions
Device or Material: Auto-cross-linked HA
Contact Duration: Absorbed
Dose: 3 m
Frequency/Duration: Single administration
Response: Bleeding, Infection
Patient characteristics (gender, mean age): HA only: 31.7 years, all female. HA plus IUD: 31.7 years, all female. IUD
only: 33.9 years, all female.
Number per group: HA only: n=30, HA plus IUD: n=24, IUD only: n=35
Observed adverse effects: “None of the 89 patients showed excessive bleeding, infection, or other complications.”

Material Performance Study - Hyaluronic Acid
|
136
Timing of adverse effects: NR
Factors that predict response: “The endometrium after therapy and the clinical pregnancy rate had a superior trend
toward in the HA group than the IUD group. In our opinion, IUDs may induce an excessive inflammatory
reaction that would cause IUA recurrence and thin the endometrium. Physical barriers such as HA gel are
interposed between adjacent injured surfaces to avoid direct contact after surgery.”
Source Citation: Zheng et al. 2020
125
Study Design: SR of HA gel to prevent IUA after intrauterine operation
Device or Material: HA gel versus control
Contact Duration: NR
Dose: NR
Frequency/Duration: Single administration
Response: None reported
Patient characteristics (gender, mean age): Review of 7 RCTs involving 952 females
Number per group: HA group: n=455, Control group: n=497
Observed adverse effects: “None of the seven studies reported gel-related or surgical-related complications, including
hemorrhage, perforation or cervical laceration.”
Timing of adverse effects: NR
Factors that predict response: NR
Source Citation: Zhou et al. 2020
115
Study Design: Single-center, double-blind RCT of females with moderate to severe IUAs
Device or Material: Auto-cross linked HA gel (MateRegen gel) versus control
Contact Duration: NR
Dose: 3 m
Frequency/Duration: Single administration
Response: Change in menstrual pattern
Patient characteristics (gender, mean age): 145 females. HA group: mean age 31.9 years, Control group: mean age
31.9 years.
Number per group: HA group: n=122, Control group: n=123
Observed adverse effects: No surgical complications were reported for the HA and control groups. The change in the
menstrual pattern after surgery in the treatment group (87.7%, 107/122) was not significantly different (p
= .064) from that of the control group (76.4%, 97/123).
Timing of adverse effects: 1, 2 and 3 months
Factors that predict response: NR
Source Citation: Fei et al. 2019
126
Study Design: SR of HA gel to prevent IAU formation after hysteroscopic adhesiolysis

Material Performance Study - Hyaluronic Acid
|
137
Device or Material: HA gel versus control
Contact Duration: NR
Dose: NR
Frequency/Duration: Single administration
Response: None reported
Patient characteristics (gender, mean age): Review of 2 RCTs and 4 clinical studies involving 394 females
Number per group: HA group: n=176, Control group: n=218
Observed adverse effects: No complications were reported for the HA and control groups.
Timing of adverse effects: NR
Factors that predict response: NR
Source Citation: Kim et al. 2019
112
Study Design: Single-center RCT of patients undergoing laparoscopic radical cystectomy
Device or Material: HA+CMC gel (Guardix-sol, Hanmi Medicare, Seoul, South Korea)
Contact Duration: NR
Dose: 5 g evenly applied on whole the lower abdominal cavity including the anastomotic site using the spray
instrument
Frequency/Duration: Single administration
Response: Adhesive bowel obstruction, Rectal injury from surgery, Other complications not described
Patient characteristics (gender, mean age): Guardix-sol group: 64.6 years, 32 male, 6 female, Control group: 68.6
years, 32 male, 6 female
Number per group: Guardix-sol group: n=38, Control group: n=38
Observed adverse effects: One patient in the experimental group suffered a rectal injury during surgery. 8 patients in
the Guardix-sol group and 20 patients in the control group “had postoperative complications other than
blood transfusion. Complication rates did not differ significantly in the experimental and control groups,
except for adhesive bowel obstruction, which occurred in zero and six patients, respectively (p = 0.025).”
Timing of adverse effects: NA
Factors that predict response: “High molecular weight HA is hydrophilic, non-immunogenic, and viscoelastic, enabling
it to coat the mucosal surface and have lubricating activity. HA reduces or prevents trauma in surgical
patients due to its physical properties.”
Source Citation: Lee et al. 2019
109
Study Design: Single-center RCT of patients undergoing colorectal surgery
Device or Material: HA+CMC barrier (Seprafilm) or HA+CMC gel (Guardix) versus control
Contact Duration: Absorbed
Dose: 5 g of Guardix
Frequency/Duration: Single administration
Response: Small bowel obstruction rate, Pneumonia, SSI, Anastomosis leakage, Intra-abdominal abscess
Material Performance Study - Hyaluronic Acid
|
138
Patient characteristics (gender, mean age): Seprafilm group: 63.2 years, 103 male, 64 female, Guardix group:60.3
years, 87 male, 68 female, Control group: 62.5 years, 87 male, 78 female
Number per group: Seprafilm group: n=167, Guardix group: n=155, Control group: n=166
Observed adverse effects: “The overall complication rates did not differ significantly between the Guardix and
Seprafilm groups (12.9% vs. 15.5% vs. 18.7%; P = 0.533.” Complications included pneumonia, SSI,
anastomosis leakage, intra-abdominal abscess; all related to surgery.“Small bowel obstruction developed in
9 patients (5.8%) in the Guardix group and 9 patients (7.1%) in the Seprafilm group and 19 patients
(11.4%) in the control group.”
Timing of adverse effects: NR
Factors that predict response: NR
Source Citation: Li et al. 2019
116
Study Design: Multi-center, double-blind RCT of females undergoing dilation and curettage
Device or Material: NCH gel (MateRegen) versus control
Contact Duration: NR
Dose: 3 m
Frequency/Duration: Single administration
Response: None reported
Patient characteristics (gender, mean age): 274 females. MateRegen group: mean age 26.94 years, age range 17-44
years. Control group: mean age 26.84 years, age range 19-45 years.
Number per group: MateRegen group: n=137, Control group n=137
Observed adverse effects: “No serious adverse events were observed during the study period, and there were no
prolonged hospitalizations or reoperations owing to adverse events. Furthermore, no adverse events were
attributed to the NCH gel treatment.” “No signs of postoperative infection were reported in either group.”
Timing of adverse effects: 3 months
Factors that predict response: NR
Source Citation: Saito et al. 2019
107
Study Design: Single-center RCT of patients who underwent elective colectomy for colon cancer
Device or Material: HLA+CMC barrier (Seprafilm) versus control
Contact Duration: Absorbed in 7 days
Dose: 2 sheets (12.7 cm × 14.7 cm)
Frequency/Duration: Single administration
Response: SSI, Suture failure, Respiratory complications, Cardiovascular complications
Patient characteristics (gender, mean age): Seprafilm group: median age 69 years, 90 male, 76 female, Control
group: median age 70 years, 98 male, 81 female
Number per group: Seprafilm group: n=166, Control group: n=179

Material Performance Study - Hyaluronic Acid
|
139
Observed adverse effects: “As for perioperative complications, surgical‐site infection (1.2% in the seprafilm group
and 3.4% in the control group), suture failure (3.6% and 6.7%), and respiratory complications (1.2% and
0%) and cardiovascular complications (1.2% and 0.6%) did not differ significantly between the groups.”
Timing of adverse effects: Perioperative
Factors that predict response: NR
Source Citation: Can et al. 2018
117
Study Design: Single-center RCT of females who underwent curettage for retained placental tissue
Device or Material: NCH gel (MateRegen) versus control
Contact Duration: Absorbed
Dose: 5 mL NCH gel
Frequency/Duration: Single administration
Response: None reported
Patient characteristics (gender, mean age): NCH gel group: 30.1 years, all women, Control group: 28.9 years, all
women
Number per group: NCH gel group: n=20, Control group: n=28
Observed adverse effects: “No complications or adverse events associated with intrauterine application of gel were
reported in the intervention group, and no complications related to curettage or follow-up hysteroscopy
were observed in either group.”
Timing of adverse effects: NR
Factors that predict response: NR
Source Citation: Farag et al. 2018
1
Study Design: SR of HA gel to prevent IAU formation after laparoscopic gynecologic surgery
Device or Material: HA gel (Hyalobarrier Gel) versus control
Contact Duration: NR
Dose: NR
Frequency/Duration: Single administration
Response: Adhesions
Patient characteristics (gender, mean age): One RCT involving 43 females. Patient age NR
Number per group: HA group: n=25, Control group: n=18
Observed adverse effects: “Hyalobarrier Gel placement and “full conditioning” resulted in a significantly decreased
incidence of adhesions compared with standard laparoscopy (25% and 100%, respectively; p = .0005).”
Timing of adverse effects: NR
Factors that predict response: “Pain was associated with adhesions, as shown by conscious laparoscopic pain
mapping. Filmy mobile adhesions resulted in the highest verbal pain scores followed by dense mobile
adhesions, filmy fixed adhesions, and dense fixed adhesions. Pain scores were highest when adhesions were
between a segment of bowel and an ovary.”

Material Performance Study - Hyaluronic Acid
|
140
Source Citation: Hooker et al. 2018
129
Study Design: Multi-center, double-blind RCT of females undergoing dilation and curettage
Device or Material: Auto-cross-linked HA gel versus control
Contact Duration: NR
Dose: 10 mL HA gel
Frequency/Duration: Single administration
Response: None reported
Patient characteristics (gender, mean age): 143 females. HA group: mean age 34.1 years, age range 20-44 years.
Control group: mean age 33.6 years, age range 19-44 years.
Number per group: HA group: n=73, Control group: n=70
Observed adverse effects: No complications were reported for the HA and control groups.
Timing of adverse effects: NR
Factors that predict response: NR
Source Citation: Liu et al. 2018
127
Study Design: SR of HA gel to prevent IAU formation after gynecologic surgery
Device or Material: HA gel versus control
Contact Duration: NR
Dose: NR
Frequency/Duration: Single administration
Response: Discomfort, Uterine perforation
Patient characteristics (gender, mean age): Review of 6 RCTS involving 564 females. HA group: mean age 32.8
years. Control group: mean age 32.8 years.
Number per group: HA group: n=286, Control group: n=278
Observed adverse effects: “Only one trial, conducted by Hooker et al. [9], reported complications associated with use
of hyaluronic acid gel. The major complications were patient discomfort and uterine perforation, with no
statistically significant differences between the 2 groups.”
Timing of adverse effects: NR
Factors that predict response: NR
Source Citation: Yan and Xu 2018
121
Study Design: SR of adjuvants to prevent IAU formation after gynecologic surgery
Device or Material: Auto–cross-linked HA gel and HA+CMC barrier versus control
Contact Duration: NR
Dose: NR
Frequency/Duration: Single administration

Material Performance Study - Hyaluronic Acid
|
141
Response: None reported
Patient characteristics (gender, mean age): Review of 7 RCTs involving 748 females. Patient age NR for all RCTs.
Number per group: NR
Observed adverse effects: Patient complications were not reported for the included RCTs.
Timing of adverse effects: 1 month to 8 months
Factors that predict response: NR
Source Citation: Hooker et al. 2017
130
Study Design: Multi-center, double-blind RCT of females undergoing dilation and curettage
Device or Material: Auto-cross-linked HA gel
Contact Duration: NR
Dose: 10 mL HA gel
Frequency/Duration: Single administration
Response: Cervix laceration, Excessive bleeding, Infection, Pain, Uterus perforation
Patient characteristics (gender, mean age): 149 females. HA group: mean age 35.0 years, age range 20-44 years.
Control group: mean age 33.9 years, age range 19-44 years.
Number per group: HA group: n=77, Control group: n=72
Observed adverse effects: Cervix laceration was experienced by one patient in the HA group (1.3%) and no patients
in the control group (p=1.00). Three patients in the HA group had excessive bleeding (3.9%) compared to
one patient in the control group (1.4%) (p=0.62). Two patients in the HA group had postoperative
infections (2.6%) compared to one patient in the control group (1.4%) (p=1.00). Four patients in the HA
group had postoperative pain (5.2%) compared to three patients in the control group (4.2%) (p=1.00).
Uterus perforation was experienced by one patient in the HA group (1.3%) and no patients in the control
group (p=1.00).
Timing of adverse effects: 10 weeks
Factors that predict response: NR
Source Citation: Healy et al. 2016
122
Study Design: SR of postoperative prevention measures on IAU formation after operative hysteroscopy
Device or Material: HA gel versus control, HA+CMC gel versus control
Contact Duration: NR
Dose: 10 ml HA gel, 10 ml HA+CMC gel
Frequency/Duration: Single administration
Response: None reported
Patient characteristics (gender, mean age): Review of 3 clinical studies involving 262 females. Patient age for HA
groups NR. HA+CMC and HA+CMC control groups: age range 18-65 years.
Number per group: HA group: n=112, HA control group: n=110, HA+CMC group: n=18, HA+CMC control group:
n=22

Material Performance Study - Hyaluronic Acid
|
142
Observed adverse effects: No complications were reported for the HA, HA control, HA+CMC, or HA+CMC control
groups.
Timing of adverse effects: 9 weeks to 3 months
Factors that predict response: NR
Source Citation: Kiefer et al. 2016
108
Study Design: Multicenter, single (patient)-blinded, RCT of women undergoing caesarean delivery
Device or Material: HA+CMC barrier (Seprafilm) versus control
Contact Duration: Absorbed in 7 days
Dose: 1 sheet
Frequency/Duration: Single administration
Response: Postoperative complications (ileus, abscess formation, wound complication Bowel or bladder injury,
hysterectomy, blood transfusion, and uterine rupture or dehiscence
Patient characteristics (gender, mean age): 753 females. HA+CMC group: 30.4 years, Control group: 30.9 years
Number per group: HA+CMC group: n=380, Control group: n=373
Observed adverse effects: HA+CMC vs. Control Any wound complication: 2.1% vs. 0.8%, p = 0.22, RR = 2.8(0.7 –
11.0)
Timing of adverse effects: 6 to 8 weeks
Factors that predict response: “Routine use of a HA-CMC adhesion barrier did not reduce the incidence of adhesions
at the time of subsequent cesarean delivery. Similarly, no differences in operative times or the incidence of
complications were identified.” “With regard to the safety of HA-CMC, we did not identify any short-term
safety concerns. While not specifically powered for safety data, the large number of enrolled patients, the
lack of adverse events, and similar postoperative course for both groups are reassuring.”
Source Citation: Hindocha et al. 2015
123
Study Design: SR of solid agents, gel agents, liquid agents and pharmacological agents, used as adjuvants to prevent
formation of adhesions after gynecologic surgery
Device or Material: HA+CMC barrier versus control
Contact Duration: NR
Dose: NR
Frequency/Duration: Single administration
Response: Adverse outcomes, Pelvic pain (new pain, change in severity of pain)
Patient characteristics (gender, mean age): Review of two SRs with 47 RCTs. “Patient characteristics were reported
inconsistently across the studies in the reviews.”
Number per group: Between 59 and 127 females in HA+CMC and control groups
Observed adverse effects: “Ahmad 2014(a) identified only one trial that investigated the adverse effects of sodium
hyaluronate and carboxymethylcellulose, with a total of 59 participants treated with the agent. No adverse
effects secondary to the agent were reported.” “No review found any trials that investigated the effect of
sodium hyaluronate and carboxymethylcellulose on postoperative pelvic pain.”
Material Performance Study - Hyaluronic Acid
|
143
Timing of adverse effects: NR
Factors that predict response: NR
Source Citation: Liu et al. 2015
118
Study Design: Multi-center, reviewer blinded, RCT of patients undergoing laparoscopic surgery for the primary
removal of adhesion, myoma, ovarian cyst, or endometriotic cyst
Device or Material: NCH gel (HyaRegen NCH gel) versus saline placebo
Contact Duration: NR
Dose: 160 ml NCH gel or saline
Frequency/Duration: Single administration
Response: Adverse events were noted but not described
Patient characteristics (gender, mean age): 196 females, age range 18-45 years
Number per group: NCH group: n=102, Saline group: n=94
Observed adverse effects: “During the study period, no adverse events were attributed to the NCH gel treatment. No
serious adverse events were observed. The adverse events were mostly mild, spontaneously resolved, and
comparable in the 2 groups.”
Timing of adverse effects: 3 days, 30 days, 9 weeks
Factors that predict response: “In a preclinical animal study, the maximum volume of NCH gel administered without
any adverse effects was at least 15-fold higher than that applied in this study (unpublished data).” “Owing
to the high safety threshold, 160 mL of NCH gel was applied to the abdominopelvic cavity to provide broad
coverage on the organ and tissue surfaces that sustained surgical trauma as well as their adjacent and
suspected adhesiogenic surfaces, and thus a global effect on reduced adhesion formation throughout the
cavity. In the contrast, the doses for most site-specific gels are within 40 mL because of safety and/or cost
concerns”.
Source Citation: Hu et al. 2015
132
Study Design: Single-center, double blind, RCT of patients undergoing colorectal surgery with a defunctioning
ileostomy or colostomy
Device or Material: HA gel or chitosan versus control
Contact Duration: HA is absorbed
Dose: NR
Frequency/Duration: Single administration
Response: Post-operative short-term complications: peristomal curaneous infection, intra-abdominal hemorrhage,
abscess Long-term postoperative complications: intestinal obstruction, anastomotic leakage
Patient characteristics (gender, mean age): HA gel group: 50.3 years, 52% male, Chitosan group: 53 years, 31%
male, Control group: 51 Years, 72% male
Number per group: HA gel group: n=29, Chitosan group: n=29, Control group: n=29
Observed adverse effects: No postoperative complications, 30 days. Long-term complications were similar for each
group: Sodium hyaluronate gel 10.5%, chitosan 16.2%, control 17.9%.
Timing of adverse effects: See above. Longest follow-up was 42 months.

Material Performance Study - Hyaluronic Acid
|
144
Factors that predict response: NR
Source Citation: Berdah et al. 2014
110
Study Design: Multicenter, single (patient)-blinded, RCT of patients undergoing laparoscopic colorectal and/or small
bowel surgery
Device or Material: HA+CMC powder (Sepraspray Adhesion Barrier) versus no adhesion barrier
Contact Duration: HA+CMC powder is completely absorbed in less than 28 days
Dose: 1 to 10 grams, mean ± SD amount of powder applied was 2.7 ± 1.4 g
Frequency/Duration: Single administration
Response: Serious adverse events: pelvic abscess, abdominal abscess, SSI, Anastomotic fistula, Peritonitis
Patient characteristics (gender, mean age): HA+CMC group: 57.6 years, 50.5% male. Control group: 56.1 years,
49% male
Number per group: HA+CMC group: n=105, Control group: n=104
Observed adverse effects: Any adverse event: HA+CMC 62.9%, control 39.4%, p <0.001. At least 1 serious adverse
event: HA+CMC 27.6%, control 10.6%, p <0.001. Pelvic abscess: HA+CMC 4.8%, control 1.9%. Abdominal
abscess: HA+CMC 3.8%, control 0%. Ileus: HA+CMC 2.9%, control 0%. “At least one SSI was experienced
by 22/105 (21%) of patients in the HA/CMC powder group versus 15/104 (14%) in the no adhesion barrier
group (P = 0.216), and at least one serious SSI by 13/105 (12%) versus 9/104 (9%), respectively (P =
0.38).” HA/CMC versus control: serious SSIs of pelvic abscess (4.8% and 1.9%, respectively), anastomotic
fistula (2.9% and 3.8%), and peritonitis (1.9% and 2.9%) were not statistically different.
Timing of adverse effects: Study assessment was 28 to 35 days post-surgery. HA+CMC powder is completely
absorbed in less than 28 days.
Factors that predict response: There was no relationship between the amount of HA+CMC powder used and
incidence of adverse events or serious adverse events. “The probability of a serious adverse event was
greater in the HA/CMC powder versus the no adhesion barrier group (OR = 4.08; 95% CI, 1.67–9.95; P =
0.002), in younger patients (for age in years, OR = 0.94; 95% CI, 0.91–0.98; P = 0.002), and in patients
who smoked frequently (OR = 1.06; 95% CI, 1.02–1.10; P = 0.006).” “The powder formulation of HA/CMC
used in this study is likely to be an important contributing factor for the increased frequency of adverse
events and serious adverse events.” “Due to the potential for greater diffusion of the powder formulation,
the authors speculate that migration away from the application site to anastomoses could have occurred in
some cases. Furthermore, over-hydration of the HA/CMC powder might have resulted in pooling of the
resulting gel away from the application site, raising the possibility of migration onto an anastomosis or
provision of a nidus for abscess; such migration to anastomoses might increase the rate of SSIs.”
Source Citation: Chung et al. 2013
113
Study Design: Single-center RCT of patients undergoing surgery for chronic epididymitis
Device or Material: HA+CMC barrier (Guardix-sol) versus control
Contact Duration: Evaluated at 24 weeks
Dose: 3 g
Frequency/Duration: Single administration
Response: None reported
Patient characteristics (gender, mean age): HA+CMC group: 56.6 years, all male. Control group: 58.5 years, all male

Material Performance Study - Hyaluronic Acid
|
145
Number per group: HA+CMC group: n=22, Control group: n=21
Observed adverse effects: There were no postoperative complications such as wound infection or hematoma in either
group. HA+CMC was not responsible for any adverse events. HA+CMC was effective at reducing pain
associated with epididymitis.
Timing of adverse effects: NR
Factors that predict response: NR
Source Citation: Dupré et al. 2013
106
Study Design: Multi-center RCT of metastatic colorectal cancer patients requiring 2-stage hepatectomy
Device or Material: HA+CMC barrier (Seprafilm) versus control
Contact Duration: Up to 90 days after surgeries
Dose: 4 sheets (12.7 × 15.2 cm
2
)
Frequency/Duration: Single administration
Response: Complications, Mortality, Parietal abscess, Right portal vein embolization, Small bowel obstruction
Patient characteristics (gender, mean age): First-stage Seprafilm group: 19 male, 22 female, mean age 61 years, age
range 40-79 years. First-stage Control group: 11 male, 2 female, mean age 55 years, age range 48-76
years. Second-stage Seprafilm and Control group patient characteristics NR.
Number per group: First-stage Seprafilm group: n=41, First-stage Control group: n=13, Second-stage Seprafilm
group: n=30, Second-stage Control group: n=11
Observed adverse effects: “No patient died within 90 days of the first-stage hepatectomy. The overall complication
rate was 48.8% in the HA membrane group [First-stage Seprafilm group] and 30.8% among controls [First-
stage Control group].” “Eighteen patients had a grade I or II complication (36.6% in the HA membrane
group [First-stage Seprafilm group] and 23.1% in controls [First-stage Control group]). Most of these minor
complications were related to urinary tract infections, minor pleural effusion, blood transfusion, or
positioning of a gastric tube. The rate of parietal abscess was low (2.4% in the HA membrane group [First-
stage Seprafilm group] and 15.4% in controls [First-stage Control group]). Seven patients (5 in the HA
membrane group [First-stage Seprafilm group] and 2 controls [First-stage Control group]) had postoperative
right portal vein embolization.” Seven patients had at least one complication in the Second-stage Seprafilm
group (23.3% and six patients had at least one complication in the Second-stage Control group (p=0.07).
“One patient in each [Second-stage] group required a percutaneous drainage for a biloma (grade IIIa), and
2 patients in the [Second-stage] control group had a reoperation (grade IIIb), one for a small bowel
obstruction and the other for a major parietal abscess.”
Timing of adverse effects: 30 days and 90 days after surgeries
Factors that predict response: “Although the immediate postoperative complication rate was somewhat higher in the
HA membrane group, this was due to nonspecific events unrelated to its use or to liver surgery.”
Source Citation: Ouaïssi et al. 2012
105
Study Design: SR of surgical literature published between 1995 and 2009 on post-operative adhesions after
gastrointestinal surgery
Device or Material: Seprafilm
Contact Duration: NR
Dose: NR

Material Performance Study - Hyaluronic Acid
|
146
Frequency/Duration: Single administration
Response: Post-operative adhesions, Intestinal fistula
Patient characteristics (gender, mean age): 5 RCTs: Fazio et al. . Reduction in adhesive small-bowel obstruction by
Seprafilm(registered trademark) adhesion barrier after intestinal resection. Dis Colon
Number per group: Rectum 2006;49(1):1—11. RCT, n = 1,791. Kunosoki et al. Bioresorbable hyaluronate-
carboxymethylcellulose membrane (Seprafilm) in surgery for rectal carcinoma: A prospective randomized
clinical trial. Surgery Today 2005;35(11):940—5. RCT, n = 62. Becker et al... Prevention of postoperative
abdominal adhesions by a sodium hyaluronate based bioresorbable membrane: a prospective, randomized,
double-blind multicenter study. J Am Coll Surg 1996;183(4):297—306. RCT, n = 183. Tang et al. .
Bioresorbable adhesion barrier facilitates early closure of the defunctioning ileostomy after rectal excision: a
prospective, randomized trial. Dis Colon Rectum 2003;46(9):1200—7. RCT, n = 70. Vrijland et al. Fewer
intraperitoneal adhesions with use of hyaluronic acid carboxymethylcellulose membrane: A randomized
clinical trial. Ann Surg 2002;235(2):193—9. RCT, n = 71.
Observed adverse effects: “As regards adverse effects, the use of Seprafilm has not been shown to result in a
significant increase in morbidity, but it must be noted that when Seprafilm was placed in direct contact with
intestinal anastomoses, a significant increase in the rate of intestinal fistulas and their secondary morbidity
was observed.”
Timing of adverse effects: NR
Factors that predict response: “In sum, there are sufficient studies with an adequate level of evidence to prove that
the use of Seprafilm allows a significant reduction in the number, extent, and severity of post-operative
adhesions, both at the incisional interface and at the operative site.
Source Citation: Mais et al. 2012
119
Study Design: SR of HA gel use to prevent intraperitoneal adhesion and IUA after endoscopic gynecological surgery
Device or Material: Auto-cross-linked HA gel (Hyalobarrier) versus control
Contact Duration: Up to 3 months
Dose: NR
Frequency/Duration: Single administration
Response: Abdominal complaints (nausea, vomiting), Nausea, Vomiting
Patient characteristics (gender, mean age): Review of 5 RCTs involving 335 females. Patient characteristics NR for all
RCTs.
Number per group: Hyalobarrier group: n=167, Control group: n=168
Observed adverse effects: “A total of six adverse events (three in gel [Hyalobarrier] groups and three in control
groups) were reported in the two RCTs performed in laparoscopy. In one trial two patients had nausea and
one patient had vomiting in gel [Hyalobarrier] group and one patient had nausea in control group [8]. No
adverse events were reported in the three RCTs performed in hysteroscopy [16,17,19].”
Timing of adverse effects: Two or three months
Factors that predict response: NR
Source Citation: Fossum et al. 2011
111
Study Design: Multicenter, double-blinded, RCT of patients undergoing laparoscopic myomectomy

Material Performance Study - Hyaluronic Acid
|
147
Device or Material: HA+CMC barrier (Sepraspray Adhesion Barrier)
Contact Duration: NR
Dose: NR
Frequency/Duration: Single administration
Response: Adverse events, Deep vein thrombosis, Intra-abdominal abscess, SSI rate
Patient characteristics (gender, mean age): 41 females. Sepraspray group: mean age 36 years. Control group: mean
age 37 years.
Number per group: Sepraspray group: n=21, Control group: n=20
Observed adverse effects: “No overall difference was noted in the number of patients with adverse events in either
the control (n=12; 60% of patients) or Sepraspray Adhesion Barrier treated (n=14; 67% of patients)
groups. No adverse event directly related to Sepraspray Adhesion Barrier was identified by the surgeons,
nor was there any report of surgical site infection, intra-abdominal abscess formation, or deep vein
thrombosis.”
Timing of adverse effects: 1 week, 1 month
Factors that predict response: NA
Source Citation: van der Wal et al. 2011
150
Study Design: RCT of patients requiring Hartmann’s procedure for sigmoid diverticulitis or obstructed rectosigmoid,
long-term follow-up
Device or Material: Seprafilm under the midline and in the pelvis during laparotomy or no barrier
Contact Duration:
Dose: NR
Frequency/Duration: Single administration
Response: Abdominal complaints (pain, nausea, obstipation)
Patient characteristics (gender, mean age): Seprafilm: 66.9 years, 65% male, Control: 67.8 years, 44% male
Number per group: Seprafilm: 16, Control: 19
Observed adverse effects: “Analysis of the questionnaire revealed that abdominal complaints (pain, nausea,
obstipation [severe form of constipation]) occurred significantly less often in the Seprafilm group than in the
control group: 6 patients (35%) in the Seprafilm group experienced at least 1 episode of abdominal
complaints of 3 months or longer, whereas 14 patients (78%) in the control group went through at least 1
episode of abdominal complaints of 3 months or longer (P = 0.018).”
Timing of adverse effects: Follow-up: Seprafilm 96 months (11 – 118), control 98 months (19 – 119)
Factors that predict response: NR
Source Citation: Zhao et al. 2021
104
Study Design: SR of Seprafilm use to prevent intestinal obstruction after gastrointestinal neoplasms surgery
Device or Material: Seprafilm versus control
Contact Duration: NR
Dose: 1 sheet

Material Performance Study - Hyaluronic Acid
|
148
Frequency/Duration: Single administration
Response: Aspartate aminotransferase concentration, Alanine aminotransferase concentration, Blood urea nitrogen
concentration, Creatinine concentration, WBC count
Patient characteristics (gender, mean age): Review of 2 RCTs and 1 clinical study involving 750 patients. Seprafilm
group: 214 male, 137 female. Control group: 231 male, 168 female.
Number per group: Seprafilm group n=351, Control group n=399
Observed adverse effects: WBC counts and concentrations of aspartate aminotransferase, alanine aminotransferase,
and blood urea nitrogen were not significantly different between groups on POD5 and 7. On POD 5, serum
creatinine was significantly higher in the Seprafilm group than the control group (WMD, 0.15; 95% CI,
0.05–0.25; I
2
=78%; p=.003), but there was not a significant difference between groups on POD 7.
Timing of adverse effects: POD 5 and 7
Factors that predict response: The authors noted “that Seprafilm did not cause acute postoperative inflammation in
short term and had almost no effect on the liver and renal function of the patients.”
Source Citation: Kiefer et al. 2016
108
Study Design: Multicenter, single (patient)-blinded, RCT of women undergoing caesarean delivery
Device or Material: HA+CMC barrier (Seprafilm) compared with no treatment
Contact Duration: Absorbed in 7 days
Dose: 1 sheet
Frequency/Duration: Single administration
Response: Fever
Patient characteristics (gender, mean age): HA+CMC: 30.4 years, all women, No treatment: 30.9 years, all women
Number per group: HA+CMC: 380 No treatment: 373
Observed adverse effects: HA+CMC vs. no treatment, Fever: 4.5% vs. 3.0%, p = 0.34, RR = 1.5 (0.7 – 3.3)
Timing of adverse effects: 6 to 8 weeks
Factors that predict response: “With regard to the safety of HA-CMC, we did not identify any short-term safety
concerns. While not specifically powered for safety data, the large number of enrolled patients, the lack of
adverse events, and similar postoperative course for both groups are reassuring.”
Source Citation: Liu et al. 2015
118
Study Design: Multi-center, reviewer-blinded, RCT of patients undergoing laparoscopic surgery for the primary
removal of adhesion, myoma, ovarian cyst, or endometriotic cyst
Device or Material: NCH gel (HyaRegen NCH gel) versus saline placebo
Contact Duration: NR
Dose: 160 ml NCH gel or saline
Frequency/Duration: Single administration
Response: C-reactive protein concentration, Platelet count, RBC count, WBC count, Neutrophil count, Hemoglobin
concentration, Aspartate aminotransferase concentration, Alanine aminotransferase concentration, Albumin
concentration, Globin concentration, Total protein concentration, Blood urea nitrogen concentration,

Material Performance Study - Hyaluronic Acid
|
149
Creatinine concentration, Total bilirubin concentration, Glucose concentration, Potassium concentration,
Sodium concentration, Chloride concentration
Patient characteristics (gender, mean age): 196 females, age range 18-45 years
Number per group: NCH group: n=102, Saline group: n=94
Observed adverse effects: “Two adverse events from laboratory tests, defined as clinically significant changes from
baseline (WBC count and blood glucose level), were reported at 9 weeks after surgery in the control group,
whereas there were no clinical significantly changes from baseline in the NCH gel group. There were no
prolonged hospitalizations or surgeries related to the adverse events.”
Timing of adverse effects: 3 days, 30 days, 9 weeks
Factors that predict response: NR
Source Citation: Hu et al. 2015
132
Study Design: Single-center, double blind, RCT of patients undergoing colorectal surgery with a defunctioning
ileostomy or colostomy
Device or Material: HA gel or chitosan versus control
Contact Duration: HA is absorbed
Dose: NR
Frequency/Duration: Single administration
Response: WBC count, Liver function, Renal function, Liver toxicity, Renal toxicity
Patient characteristics (gender, mean age): HA gel group: 50.3 years, 52% male, Chitosan group: 53 years, 31%
male, Control group: 51 Years, 72% male
Number per group: HA gel group: n=29, Chitosan group: n=29, Control group: n=29
Observed adverse effects: “There was no significant toxicity to liver or kidney related to the use of sodium
hyaluronate gel or chitosan. Liver function, renal function, and white blood cell (WBC) levels within 2 weeks
after the initial surgery were comparable among the 3 groups.”
Timing of adverse effects: Up to 42 months
Factors that predict response: NR
Source Citation: Mais et al. 2012
119
Study Design: SR of HA gel use to prevent intraperitoneal adhesion and IUA after endoscopic gynecological surgery
Device or Material: Auto-cross-linked HA gel (Hyalobarrier) versus control
Contact Duration: Up to 3 months
Dose: NR
Frequency/Duration: Single administration
Response: Fever
Patient characteristics (gender, mean age): w of 5 RCTs involving 335 females. Patient characteristics NR for all
RCTs.
Number per group: Hyalobarrier group: n=167, Control group: n=168

Material Performance Study - Hyaluronic Acid
|
150
Observed adverse effects: In one RCT performed in laparoscopy, two patients in the Control group “had
postoperative fever (≤38.5°C).”
Timing of adverse effects: Two or three months
Factors that predict response: NR
ACH = alginate hyaluronate-carboxymethylcellulose; CMC = carboxymethylcellulose; HA = sodium hyaluronic acid; IUA =
intrauterine adhesion; IUD = intrauterine device; NA = not applicable; NCH = new crosslinked hyaluronan; NR = not
reported; Obs = observational; POD = post-operative day; Retro = retrospective; R = reliable; RBC = red blood cell; RR = risk
ratio; SR = systematic review; SSI = surgical site infection; Dose = mg/kg/day; WBC = white blood cell
Material Performance Study - Hyaluronic Acid
|
151
Table 26: Anti-adhesion: Nasal packing - Health Effect (In Vivo) Human Studies
Local Response/ Toxicity
Source Citation: Deng et al. 2018
136
Study Design: Single-center RCT of patients undergoing tympanoplasty for adhesive otitis media
Device or Material: HA (MeroGel)
Contact Duration: Absorbed in 2 weeks
Dose: Two identical pieces about 0.5 0.5 0.2 cm3 in size.
Frequency/Duration: Single administration
Response: Hearing loss, Re-otorrhea (ear discharge), Prosthesis extrusion
Patient characteristics (gender, mean age): HA: 40.8 years, 44 male, 28 female, Cartilage: 38.6 years, 25 male, 39
female, HA plus cartilage: 39.2 years, 35 male, 34 female
Number per group: HA: 72, Cartilage: 64, HA plus cartilage: 69
Observed adverse effects: “No patient needed reoperations or suffered from sensorineural hearing loss after surgery.
There were no significant differences between any two of the three groups in the rates of re-otorrhea, or
extrusion of the prosthesis.”
Timing of adverse effects: NR
Factors that predict response: “HA can be safely and easily used in clinical applications. It is inert and stays at
biologic sites over extended periods of time. Hyaluronic acid is suitable for otologic surgery because it has
some physical properties, such as not causing or even regulating inflammatory reactions, and binding water
as a viscoelastic cushion in the healing process.”
Source Citation: Chen et al. 2017
134
Study Design: SR of RCTs to investigate the influence of hyaluronan nasal dressing on the clinical outcome of ESS.
Device or Material: HA: Sepragel and MeroGel
Contact Duration: MeroGel absorbed in 2 weeks
Dose: NR
Frequency/Duration: Single administration
Response: Synechia, Infection
Patient characteristics (gender, mean age): Mean ages were 44.8, 41.5, and 39.1 years with one study not reporting.
Percent female was 22%, 6%, and 17% with one study not reporting.
Number per group: 4 RCTs n = 352
Observed adverse effects: HA nasal dressing failed to reduce synechia (adhesion in the nose) (OR 0.45 [95% CI,
0.19 –1.03]; p = 0.06), crust (OR 1.00 [95% CI, 0.20 –5.09]; p = 1.00), and infection (OR 0.84 [95% CI,
0.46 –1.53]; p = 0.56) compared with the control group in patients who underwent ESS. No adverse events
or complications were reported in 2 of the included RCTs
Timing of adverse effects: Follow-up times of 8 and 12 weeks.
Factors that predict response: “Hyaluronan is known as an ideal material for nasal dressings with excellent
biocompatibility and multifunctional role for scar-free wound healing. Results of previous studies revealed that
Material Performance Study - Hyaluronic Acid
|
152
hyaluronan could improve the reepithelialization, modulate inflammatory response and stimulate angiogenesis, and
markedly reduce fibrous scarring.”
Source Citation: Fong et al. 2017
135
Study Design: SR to evaluate endoscopic outcomes compared to standard regimens and controls for preventing post
sinus surgery complications. RCTs used in meta-analysis.
Device or Material: HA versus a control for post-endoscopic sinus surgery care. Absorbable dressing packs of
hyaluronic acid, non-absorbable dressing packs impregnated with hyaluronic acid, and topical preparations
such as nebulised ampules, sprays and creams
Contact Duration: Reabsorbed and non-resorbable
Dose: NR
Frequency/Duration: Single administration of packs. Daily application of topical sprays
Response: Adhesions
Patient characteristics (gender, mean age): Used 3 categories of HA: Absorbable dressing packs of hyaluronic acid,
non-absorbable dressing packs impregnated with hyaluronic acid, and topical preparations such as nebulised
ampules, sprays and creams.
Number per group: 13 studies n = 501 (5 double-blind RCTs, 5 single-blind RCTs, and 3 prospective comparisons)
Observed adverse effects: “Absorbable hyaluronic acid dressing preparation appears to be well tolerated, according
to patient satisfaction scores across two of the studies when compared with non-absorbable packs.”
Timing of adverse effects: ---
Factors that predict response: “It is clear that hyaluronic acid in all preparations is safe and well-tolerated by
patients, as evidenced by only one adverse event not related directly to hyaluronic acid, and the overall
satisfaction with its use in absorbable nasal packs.”
Source Citation: Shi et al. 2013
137
Study Design: RCT of patients undergoing bilateral ESS
Device or Material: PureRegen Gel Sinus (absorbable crosslinked HA hydrogel)
Contact Duration: 7 day
Dose: 2 mL
Frequency/Duration: 2 applications, second application 2 days after surgery
Response: Synechia (adhesions), Scar tissue
Patient characteristics (gender, mean age): Treated: PureRegen Gel plus gelatin sponge and Merocel (compressed,
dehydrated sponge composed of hydroxylated polyvinyl acetate). Control: Gelatin sponge and Merocel.
Treatment was randomized to sinus side in each patient. 44.8 years, 43 males and 12 females
Number per group: 55 patients
Observed adverse effects: No adverse event related to treatment was observed.
Timing of adverse effects: ---
Factors that predict response: “In this prospective, multicenter, randomized, controlled trial, data analyses suggest
PureRegen Gel Sinus as nasal dressing/packing after ESS is safe and promotes the postoperative

Material Performance Study - Hyaluronic Acid
|
153
reepithelization process and reduces the postoperative presence of synechia (obstructing and non-
obstructing), edema, crusting, and mild mucopurulent drainage.”
Source Citation: Wu et al. 2011
138
Study Design: RCT of patients with unilateral primary chronic dacryocystitis (an infection of the lacrimal sac,
secondary to obstruction of the nasolacrimal duct) undergoing endonasal endoscopic dacryocystorhinostomy
Device or Material: MeroGel (esterified derivative of hyaluronan)
Contact Duration: Reabsorbed within 2 weeks
Dose: Gel covered wound
Frequency/Duration: Single administration
Response: None reported
Patient characteristics (gender, mean age): Merogel: 41.8 years, 27 males, 85 females, Control: 40.4 years, 32
males, 83 females
Number per group: Merogel: 112, Control: 115
Observed adverse effects: None reported
Timing of adverse effects: ---
Factors that predict response: “We observed that the rate of scar formation was lower in the Merogel group, with a
lower incidence of ostial failure than in the control group. These results suggest that Merogel coverage can
significantly improve the success rate of ostial patency for EES-DCR by stimulating wound healing and
mucosa epithelialization and by preventing the formation of fibrotic tissue around the ostia.”
ESS = endoscopic sinus surgery; NA = not applicable; NR = not reported; Obs = observational; OR = odds ratio; Retro =
retrospective; R = reliable; Dose = mg/kg/day

Material Performance Study - Hyaluronic Acid
|
154
Table 27: Barrier gel for oral lesions - Health Effect (In Vivo) Human Studies
Local Response/ Toxicity
Source Citation: Al-Shammari et al. 2018
139
Study Design: RCT using split-mouth design of patients with moderate to severe chronic periodontitis
Device or Material: Gengigel HA gel
Contact Duration: Study lasted 12 weeks, unclear how long the HA remained in place
Dose: 1 mL of 0.8% HA gel
Frequency/Duration: Single subgingival application
Response: None reported
Patient characteristics (gender, mean age): 10 males, 14 females 24 to 57 years
Number per group: 24
Observed adverse effects: None reported
Timing of adverse effects:
Factors that predict response: “Hyaluronic acid showed positive effects in influencing local inflammation.”
Source Citation: Casale et al. 2016
99
Study Design: SR to examine potential effects of HA as an adjuvant treatment for chronic inflammatory disease, in
addition to its use to improve healing after common dental procedures.
Device or Material: HA
Contact Duration: Varied across studies
Dose: Topical application of 0.2% and 0.8% HA gel
Frequency/Duration: Gel application twice daily for 4 weeks was a common application.
Response: Inflammation
Patient characteristics (gender, mean age): “25 relevant publications were included, three of them regarding
gingivitis, 13 of them relating to chronic periodontitis, seven of them relating to dental surgery, including
implant and sinus lift procedures, and the remaining three articles describing oral ulcers.”
Number per group: All controlled studies.
Observed adverse effects: No adverse events observed
Timing of adverse effects:
Factors that predict response: “High-molecular-weight HA suppresses the immune response preventing excessive
exacerbations of inflammation.” “As a consequence of the many functions attributed to HA, advances have
been made in the development and application of HA-based biomaterials in the treatment of various
inflammatory conditions.” “HA promotes a remission of symptoms, not only in the marginal gingiva, but also
in the deeper-seated periodontal tissues, via the known mechanisms established for hyaluronan in wound
healing.”
Source Citation: Sapna and Vandana 2011
140

Material Performance Study - Hyaluronic Acid
|
155
Study Design: RCT using split-mouth + cross-over (mixed-design) study of patients with plaque-induced gingivitis
Device or Material: Gengigel HA gel
Contact Duration: 21 days
Dose: 0.2% HA
Frequency/Duration: Twice daily topical application for 21 days. Intrasulcular applied on alternate days.
Response: Inflammation
Patient characteristics (gender, mean age): Quadrants: Control; Scaling and topical HA gel; Only topical HA gel;
Topical and intrasulcular HA gel. 18-35 years; gender not reported
Number per group: 28
Observed adverse effects: “There was a reduction in inflammatory infiltrates from baseline to day 21 in all the
groups. The reduction was higher in the scaling + topical HA group (50%), followed by the scaling group
(44%), topical + intrasulcular HA group (33.34%), and the topical group (16.67%).” “No adverse effects to
the gel were observed on clinical examination and as reported by the patients.”
Timing of adverse effects: ---
Factors that predict response: ---
NA = not applicable; NR = not reported; Obs = observational; Retro = retrospective; R = reliable; Dose = mg/kg/day

Material Performance Study - Hyaluronic Acid
|
156
Table 28: Bulking Agents - Health Effect (In Vivo) Human Studies
Local Response/ Toxicity
Source Citation: Salih et al. 2021
141
Study Design: RCT of pediatric patients with grades III and
IV primary VUR
Device or Material: Dx/HA
Contact Duration:
Dose: 1 mL
Frequency/Duration: Single administration, endoscopic injection
Response: Obstruction, Urine leak, UTI, Renal scarring
Patient characteristics (gender, mean age): Dx/HA: median 59 months, 33% male, Surgery: median 52 months, 30%
male
Number per group: Dx/HA: 30, Surgery: 30
Observed adverse effects: Dx/HA “early postoperative complications (within the first 2 weeks postoperatively) such
as fever was recorded in 3 cases (10%) and obstruction in 1 case (3.3%) was managed by double J stent
insertion, whereas in [surgery] fever was recorded in 6 cases (20%) and urine leak in 2 cases (6.6%); there
was a statistically significant difference between the two groups (P = .003).” “As regards late complication,
in [Dx/HA], UTI was recorded in 6 cases (20%) (febrile UTI in 2 cases [6.7%] and nonfebrile UTI in 4 cases
[13.3%]). The need for CAP was recorded in 2 patients (6.7%) and new renal scarring in 2 patients (6.7%).
In [surgery], UTI was recorded in 5 cases (16.7%) (febrile in 3 cases [10.2%] and nonfebrile UTI in 2 cases
[6.5%]). The need for CAP was recorded in 2 patients (6.7%) and new renal scarring in 2 patients (6.7%).”
Timing of adverse effects: Patients were followed for 1 year.
Factors that predict response: NR
Source Citation: Kirchin et al. 2017
143
Study Design: SR to assess the effects of periurethral or transurethral injection therapy on the cure or improvement
of urinary incontinence in women. Include 1 study using HA
Device or Material: HA with dextranomer (Zuidex™) was used in 1 study published in 2009
Contact Duration: 12-month follow-up
Dose: NR
Frequency/Duration: NR
Response: Injection site complications: pain, abscesses
Patient characteristics (gender, mean age): A single trial compared mid-urethral HA with bladder neck collagen
(Lightner 2009). All women
Number per group: A total of 344 women were included in this 2:1 RCT of Zuidex via the Implacer device versus
transurethral bladder neck collagen (Lightner 2009).

Material Performance Study - Hyaluronic Acid
|
157
Observed adverse effects: “Dextranomer hyaluronic acid compound treated patients appeared to have significantly
higher rates of injection site complications (16% with the hyaluronic acid compound versus none with
collagen; RR 37.78, 95% CI 2.34 to 610.12) and this product has now been withdrawn from the market.”
Concerns about high levels of pseudo-abscess formation have led to Zuidex’s withdrawal from the market.
“Adverse events were more frequent in the Zuidex™ group: 68% versus 50% in the collagen-treated
patients (RR 1.35, 95% CI 1.10 to 1.64). Retention, dysuria and urinary tract infection rates were similar for
the two groups but micturition urgency (11% Zuidex™, 4.3% collagen) and injection site pain (8.4%
Zuidex™, 2.6% collagen) were more frequently reported in the Zuidex™ group. Injection site sterile abscess
(8.4%), injection site mass (4.4%) and pseudo-cyst formation (2.2%) were only seen in the Zuidex™ group
(RR for injection site complications 37.78, 95% CI 2.34 to 610.12); 28 of the 36 patients with periurethral
collections required secondary outpatient drainage procedures.
Timing of adverse effects: NR
Factors that predict response: NR
Source Citation: Graf et al. 2011
142
Study Design: International, multicenter, double-blind, sham controlled RCT in patients with fecal incontinence
Device or Material: NASHA Dx (Q-Med AB, Uppsala, Sweden)
Contact Duration: Up to 12 months
Dose: 1 mL of NASHA Dx was injected through an anoscope into each quadrant of the submucosa, roughly 5 mm
above the dentate line. Injected volume of NASHA Dx for patients in the active treatment group was 4–8
mL.
Frequency/Duration: Patients with continuing symptoms were given follow-up injections at 1 month.
Response: Prostatic abscess, Rectal abscess, Proctalgia, Rectal hemorrhage, Diarrhea
Patient characteristics (gender, mean age): Active: 61.8 years, 90% female, Control: 60.1 years, 87% female
Number per group: Active 136, Control 70
Observed adverse effects: Two of the treatment-related adverse events were serious, both of which were in the
active treatment group. One was a prostatic abscess that resolved after antibiotic treatment and the other
was a rectal abscess that needed surgical drainage. Both these events resolved completely. Other adverse
events seen in 5% or more of the treatment group not common in the control group: Proctalgia (14%)
(pain due to a spasm of the pelvic floor muscles, the muscles of the anal sphincter, or the muscles of the
rectum), fever (8%), rectal hemorrhage (7%), diarrhea (5%). No migration, protrusion, or leakage of the
injected agent with bowel movements.
Timing of adverse effects: Within 12 months of the injections
Factors that predict response: NR
Source Citation: Salih et al. 2021
141
Study Design: RCT of pediatric patients with grades III and
IV primary VUR
Device or Material: Dx/HA
Contact Duration:
Dose: 1 mL

Material Performance Study - Hyaluronic Acid
|
158
Frequency/Duration: Single administration
Response: Fever
Patient characteristics (gender, mean age): Dx/HA: median 59 months, 33% male, Surgery: median 52 months, 30%
male
Number per group: Dx/HA: 30, Surgery: 30
Observed adverse effects: Dx/HA “early postoperative complications (within the first 2 weeks postoperatively) such
as fever was recorded in 3 cases (10%) and obstruction in 1 case (3.3%) was managed by double J stent
insertion, whereas in [surgery] fever was recorded in 6 cases (20%) and urine leak in 2 cases (6.6%); there
was a statistically significant difference between the two groups (P = .003).” “As regards late complication,
in [Dx/HA], UTI was recorded in 6 cases (20%) (febrile UTI in 2 cases [6.7%] and nonfebrile UTI in 4 cases
[13.3%]). The need for [continuous antibiotic prophylaxis] was recorded in 2 patients (6.7%) and new renal
scarring in 2 patients (6.7%). In [surgery], UTI was recorded in 5 cases (16.7%) (febrile in 3 cases [10.2%]
and nonfebrile UTI in 2 cases [6.5%]). The need for CAP was recorded in 2 patients (6.7%) and new renal
scarring in 2 patients (6.7%).”
Timing of adverse effects: Patients were followed for 1 year.
Factors that predict response: NR
Source Citation: Graf et al. 2011
142
Study Design: International, multicenter, double-blind, sham controlled RCT in patients with fecal incontinence
Device or Material: NASHA Dx (Q-Med AB, Uppsala, Sweden)
Contact Duration: Up to 12 months
Dose: 1 mL of NASHA Dx was injected through an anoscope into each quadrant of the submucosa, roughly 5 mm
above the dentate line. Injected volume of NASHA Dx for patients in the active treatment group was 4–8
mL.
Frequency/Duration: Patients with continuing symptoms were given follow-up injections at 1 month.
Response: Fever
Patient characteristics (gender, mean age): Active: 61.8 years, 90% female, Control: 60.1 years, 87% female
Number per group: Active 136, Control 70
Observed adverse effects: Two of the treatment-related adverse events were serious, both of which were in the
active treatment group. One was a prostatic abscess that resolved after antibiotic treatment and the other
was a rectal abscess that needed surgical drainage. Both these events resolved completely. Other adverse
events seen in 5% or more of the treatment group not common in the control group: fever (8%), no fever
reported in the control group. No migration, protrusion, or leakage of the injected agent with bowel
movements.
Timing of adverse effects: Within 12 months of the injections
Factors that predict response: NR
CAP = continuous antibiotic prophylaxis; Dx = dextranomer; HA = hyaluronic acid; NA = not applicable; NASHA = non-animal
stabilized hyaluronic acid; NR = not reported; Obs = observational; RCT = randomized controlled trial; Retro = retrospective;
R = reliable; Dose = mg/kg/day; VUR = vesicoureteral reflux

Material Performance Study - Hyaluronic Acid
|
159
Table 29: : Intravesical agents for bladder pain syndrome/interstitial cystitis therapy - Health Effect (In Vivo) Human Studies
Local Response/ Toxicity
Source Citation: Özkıdık 2019
145
Study Design: Double-blind RCT of bladder pain syndrome
Device or Material: Intravesical HA, CS, or HA + CS
Contact Duration: 24 moths
Dose: HA = 120 mg, CS = 80 mg
Frequency/Duration: Once a week for 6 weeks, then twice a month for 6 months, then continued once a month until
24th month.
Response: Symptomatic cardiac arrythmia, seriously elevation liver enzymes, Peripheral neuropathy, Intolerable GI
side effects, Hematuria, UTI with fever
Patient characteristics (gender, mean age): HA: 37.1 years, 16% male, CS: 37.4 years, 12% male, HA+CS: 37.2
years, 12% males
Number per group: HA: 24, CS: 24, HA+CS: 24
Observed adverse effects: “No serious adverse event requiring discontinuation of treatment was seen in any of the
participants during the 24 months follow-up period. … None of the patients in the study quit due to the side
effects of the drugs, so tolerability was good in all groups during the 24 months follow-up period.”
Timing of adverse effects:
Factors that predict response: NR
Source Citation: De Vita et al. 2013
144
Study Design: SR to assess the value of HA and HA-CS treatment in reducing the occurrence of recurrent bacterial
cystitis (RBC).
Device or Material: Intravesical instillations of HA and HA + CS
Contact Duration:
Dose: 2 studies: 40 mg HA, 2 studies: 50 ml HA 1.6% plus CS 2.0%.
Frequency/Duration: 3 studies: once a week for 4 weeks plus once a month for 4 or 5 months, 1 study: once a week
for 4 weeks plus once every 2 weeks for a month
Response: None reported
Patient characteristics (gender, mean age): Included 4 studies: 35, 27, 34, 69 years. All patients were adult women.
Number per group: 143
Observed adverse effects: The combination of HA and CS significantly improved pelvis pain. No adverse events or
complications were noted.
Timing of adverse effects:
Factors that predict response: HA and CS “decrease chronic pelvic inflammation and pain due to visceral
hypersensitivity not only by inhibiting peripheral C-fiber nociceptors and blocking smooth-muscle
contraction, but also by preventing immune-cell migration and mast-cell degranulation and extravasion.”

Material Performance Study - Hyaluronic Acid
|
160
CS = chondroitin sulphate; HA = hyaluronic acid; NA = not applicable; NR = not reported; Obs = observational; Retro =
retrospective; R = reliable; Dose = mg/kg/day

Material Performance Study - Hyaluronic Acid
|
161
Table 30: Organ spacer - Health Effect (In Vivo) Human Studies
Local Response/ Toxicity
Source Citation: Prada et al. 2016
146
Study Design: Case series of patients undergoing high-dose-rate interstitial brachytherapy for prostate cancer
Device or Material: Transperineal HA injection into the peri-rectal fat
Contact Duration: NR
Dose: NR
Frequency/Duration: Single administration
Response: Urinary tract pain, Urinary tract obstruction, Urinary retention
Patient characteristics (gender, mean age): Median 71 years
Number per group: 60 with prostate adenocarcinoma
Observed adverse effects: No adverse effects were associated with HA injection. At three months urinary tract pain
(dysuria) grade 1 occurred in 4 patients (7%). At three months later 16 patients (40%) had grade 1 urinary
tract obstruction. “No other chronic toxicity, such as incontinence, late urinary retention or urethral
narrowing has been observed after treatment.”
Timing of adverse effects: NR
Factors that predict response: NR
Source Citation: Chapet et al. 2015
147
Study Design: Case series of patients with low-risk to intermediate-risk prostate cancer undergoing hypofractionated
radiation therapy
Device or Material: HA (NASHA Spacer gel)
Contact Duration: NR
Dose: 10 mL
Frequency/Duration: Single administration injection
Response: Lower abdominal pain, Hematuria
Patient characteristics (gender, mean age): 71 years
Number per group: 36
Observed adverse effects: “At the time of injection, the mean pain score was 4.6 ± 2.3 (n = 28). Thirty minutes after
the injection, 1 patient still reported pain scored as 2/10, and 1 patient reported pain scored as 3/10, which
persisted.” One patient each experiencing lower abdomen pain, hematuria. “A hematoma developed in 1
patient between the rectum and the bladder, which had to be surgically removed.” Patients had a moderate
platelet deficit.
Timing of adverse effects: During and after injection
Factors that predict response: “At the time of injection patients reported moderate pain, which disappeared within 30
minutes. This pain was most commonly felt in the pelvic floor muscles, which are sensitive and difficult to
anesthetize with an injection of lidocaine. This pain could be reduced by optimizing the local anesthesia
procedure and adding the administration of anxiolytic medication before the injection. After the injection,
the HA was very well tolerated.”

Material Performance Study - Hyaluronic Acid
|
162
Systemic Response/ Toxicity
Source Citation: Chapet et al. 2015
147
Study Design: Case series of patients with low-risk to intermediate-risk prostate cancer undergoing hypofractionated
radiation therapy
Device or Material: HA (NASHA Spacer gel)
Contact Duration: NR
Dose: 10 mL
Frequency/Duration: Single administration injection
Response: Asthenia
Patient characteristics (gender, mean age): 71 years
Number per group: 36
Observed adverse effects: One patient experienced asthenia (abnormal physical weakness or lack of energy).
Timing of adverse effects: During and after injection
Factors that predict response: “At the time of injection patients reported moderate pain, which disappeared within 30
minutes. This pain was most commonly felt in the pelvic floor muscles, which are sensitive and difficult to
anesthetize with an injection of lidocaine. This pain could be reduced by optimizing the local anesthesia
procedure and adding the administration of anxiolytic medication before the injection. After the injection,
the HA was very well tolerated.”
HA = hyaluronic acid; NA = not applicable; NR = not reported; Obs = observational; Retro = retrospective; R = reliable; Dose
= mg/kg/day

Material Performance Study - Hyaluronic Acid
|
163
Table 31: Protective topical agent for esophageal and gastric lesions in GERD - Health Effect (In Vivo) Human Studies
Local Response/ Toxicity
Source Citation: Savarino and Scarpignato 2017
148
Study Design: Multicenter, double-blind, placebo-controlled RCT of patients with non-erosive reflux disease treated
with proton pump inhibitors
Device or Material: HA-CS (Esoxx)
Contact Duration: 2 weeks
Dose: 10 mL
Frequency/Duration: 4 times a day (after each meal and at bedtime)
Response: GI disorders, Respiratory disorders
Patient characteristics (gender, mean age): Esoxx: 63% female, 45 years, Control: 59% female, 45 years
Number per group: Esoxx: 76, Control: 78
Observed adverse effects: No serious adverse events in either group. All patients were also treated with proton pump
inhibitors. Esoxx group: 23 drug-related adverse events, 5 lead to discontinuation; 13 gastrointestinal
disorders, 4 respiratory (cough, rhinitis, throat irritation) Control: 7 gastrointestinal, 1 respiratory.
Timing of adverse effects:
Factors that predict response: Gastrointestinal adverse events may be related to the proton pump inhibitors. The
increase in respiratory adverse events is likely related to using Esoxx.
Systemic Response/ Toxicity
Source Citation: Savarino and Scarpignato 2017
148
Study Design: Multicenter, double-blind, placebo-controlled RCT of patients with non-erosive reflux disease treated
with proton pump inhibitors
Device or Material: HA-CS (Esoxx)
Contact Duration: 2 weeks
Dose: 10 mL
Frequency/Duration: 4 times a day (after each meal and at bedtime)
Response: Nervous system, Cardica, Vertigo, Hypertension, Infection
Patient characteristics (gender, mean age): Esoxx: 63% female, 45 years, Control: 59% female, 45 years
Number per group: Esoxx: 76, Control: 78
Observed adverse effects: No serious adverse events in either group. All patients were also treated with proton pump
inhibitors. Esoxx group: 23 drug-related adverse events, 5 lead to discontinuation; 3 nervous system, 1
cardiac, 1 vertigo, 1 hypertension, 1 infection. Control: 1 cardiac, 3 infections.
Timing of adverse effects:
Factors that predict response: Gastrointestinal adverse events may be related to the proton pump inhibitors. The
increase in respiratory adverse events is likely related to using Esoxx.

Material Performance Study - Hyaluronic Acid
|
164
CS = chondroitin sulphate; HA = hyaluronic acid; NA = not applicable; NR = not reported; Obs = observational; Retro =
retrospective; R = reliable; Dose = mg/kg/day

Material Performance Study - Hyaluronic Acid
|
165
Table 32: Vocal cord/fold medialization - Health Effect (In Vivo) Human Studies
Local Response/ Toxicity
Source Citation: Wang et al. 2020
149
Study Design: SR to synthesize evidence on the use of IL with HA for the treatment of UVFP.
Device or Material: HA
commercially available for IL: Hylan B gel and Restylane
Contact Duration:
Dose: “For the volume of injected HA: less than 1cc or 1cc of HA was injected in seven (50%) studies, 2cc was used
in one (7.1%) study, while no detailed information was reported in the other six studies.”
Frequency/Duration: Single administration
Response: Hematoma, Edema, Local hypersensitivity, Local inflammation which may be responsible for dysphonia,
dyspnea, and dysphagia
Patient characteristics (gender, mean age):
Number per group: 14 case series (6 studies were conducted prospectively, 4 retrospectively, and 4 without specific
mention of their study design) published between 2010 and 2018 were included in the SR, n = 442
Observed adverse effects: “Generally speaking, HA is quite safe for IL and only one patient experienced hematoma
and one patient experienced edema of the aryteno-epiglottic fold and false vocal fold in our review of 14
studies. However, Hamdan [Adverse Reaction to Restylane: A Review of 63 Cases of Injection
Laryngoplasty. Ear Nose Throat J. 2019, 98, 212–216.] and Dominguez [Inflammatory reaction to hyaluronic
acid: A newly described complication in vocal fold augmentation. Laryngoscope 2017, 127, 445–449.]
described complication rates of 4.7% and 3.8% in their studies of IL for different indications including UVFP.
The most common adverse reactions are local hypersensitivity and inflammation. The hypothesized
mechanisms for adverse reaction include (1) local hypersensitivity to the proteins incidentally produced in
the HA manufacturing process, (2) vascular compression or occlusion by the injected HA, and (3)
contamination of the injector device. Although, to date, no deaths have been reported following treatment
with IL, adverse reactions may induce local edema, erythema, induration, tenderness, abscess formation,
and decreased vocal fold pliability, among others, leading to dysphonia [difficulty in speaking], dyspnea
[difficult or labored breathing], or dysphagia [difficulty or discomfort in swallowing]. Although adverse
reactions may be treated with steroids or antibiotics, the length of adverse reaction may still range from
hours to days, weeks to months, or may even have long-term sequelae without resolution (26 months).”
Timing of adverse effects:
Factors that predict response: HA “has a simple molecular structure with no structural differences across species
from human to bacteria, and the lack of amino acids renders it non-immunogenic. In addition, the vocal fold
lamina propria compartment is a pauci-cellular layer that mostly contains the extracellular matrix and HA.
The viscoelastic properties of vocal folds after the injection of HA-based material are similar to the healthy
vocal fold in animal studies. Owing to the aforementioned safety advantages, HA has become a commonly
used material for IL.
HA = hyaluronic acid; IL = injection laryngoplasty; NA = not applicable; NR = not reported; Obs = observational; Retro =
retrospective; R = reliable; UVFP = Unilateral vocal fold paralysis; Dose = mg/kg/day

Material Performance Study - Hyaluronic Acid
|
166
Appendix E. References
1. Farag S, Padilla PF, Smith KA, Sprague ML, Zimberg SE. Management, prevention, and sequelae of adhesions in women
undergoing laparoscopic gynecologic surgery: a systematic review. J Minim Invasive Gynecol. 2018 Nov;25(7):1194-
216. Also available: http://dx.doi.org/10.1016/j.jmig.2017.12.010
. PMID: 29289627.
2. Carico RL, Aspinall SL, Good CB. Fatalities possibly associated with hyaluronic acid injections: a review of events
reported to the FDA. Drugs Ther Perspect. 2021 Mar;37(3):137-40. Also available:
http://dx.doi.org/10.1007/s40267-
020-00804-z.
3. Sedrak P, Hache P, Horner NS, Ayeni OR, Adili A, Khan M. Differential characteristics and management of pseudoseptic
arthritis following hyaluronic acid injection is a rare complication: a systematic review. J ISAKOS. 2021 Mar;6(2):94-
101. Also available: http://dx.doi.org/10.1136/jisakos-2020-000438
. PMID: 33832983.
4. Di Y, Han C, Zhao L, Ren Y. Is local platelet-rich plasma injection clinically superior to hyaluronic acid for treatment of
knee osteoarthritis? A systematic review of randomized controlled trials. Arthritis Res Ther. 2018 Jun 19;20(1):128. Also
available: http://dx.doi.org/10.1186/s13075-018-1621-0
. PMID: 29921309.
5. Li Q, Qi X, Zhang Z. Intra-articular oxygen-ozone versus hyaluronic acid in knee osteoarthritis: a meta-analysis of
randomized controlled trials. Int J Surg. 2018 Oct;58:3-10. Also available: http://dx.doi.org/10.1016/j.ijsu.2018.08.007
.
PMID: 30170178.
6. Altman RD, Bedi A, Karlsson J, Sancheti P, Schemitsch E. Product differences in intra-articular hyaluronic acids for
osteoarthritis of the knee. Am J Sports Med. 2016 Aug;44(8):2158-65. Also available:
http://dx.doi.org/10.1177/0363546515609599
. PMID: 26578719.
7. Zhao H, Liu H, Liang X, Li Y, Wang J, Liu C. Hylan G-F 20 versus low molecular weight hyaluronic acids for knee
osteoarthritis: a meta-analysis. Biodrugs. 2016 Oct;30(5):387-96. Also available:
http://dx.doi.org/10.1007/s40259-
016-0186-1. PMID: 27435213.
8. Miller LE, Fredericson M, Altman RD. Hyaluronic acid injections or oral nonsteroidal anti-inflammatory drugs for knee
osteoarthritis: systematic review and meta-analysis of randomized trials. Orthop J Sports Med. 2020 Jan
27;8(1):2325967119897909. Also available: http://dx.doi.org/10.1177/2325967119897909. PMID: 32047830.
9. Ran J, Yang X, Ren Z, Wang J, Dong H. Comparison of intra-articular hyaluronic acid and methylprednisolone for pain
management in knee osteoarthritis: a meta-analysis of randomized controlled trials. Int J Surg. 2018 May;53:103-10.
Also available: http://dx.doi.org/10.1016/j.ijsu.2018.02.065
. PMID: 29574247.
10. He WW, Kuang MJ, Zhao J, Sun L, Lu B, Wang Y, Ma JX, Ma XL. Efficacy and safety of intraarticular hyaluronic acid and
corticosteroid for knee osteoarthritis: a meta-analysis. Int J Surg. 2017 Mar;39:95-103. Also available:
http://dx.doi.org/10.1016/j.ijsu.2017.01.087
. PMID: 28137554.
11. Bannuru RR, Vaysbrot EE, Sullivan MC, McAlindon TE. Relative efficacy of hyaluronic acid in comparison with NSAIDs
for knee osteoarthritis: a systematic review and meta-analysis. Semin Arthritis Rheum. 2014 Apr;43(5):593-9. Also
available: http://dx.doi.org/10.1016/j.semarthrit.2013.10.002
. PMID: 24216297.
12. Miller LE, Bhattacharyya S, Parrish WR, Fredericson M, Bisson B, Altman RD. Safety of intra-articular hyaluronic acid for
knee osteoarthritis: systematic review and meta-analysis of randomized trials involving more than 8,000 patients.
Cartilage. 2019 Nov 16;Online ahead of print. Also available: http://dx.doi.org/10.1177/1947603519888783
. PMID:
31735075.
13. Honvo G, Reginster JY, Rannou F, Rygaert X, Geerinck A, Rabenda V, McAlindon T, Charles A, Fuggle N, Cooper C,
Curtis E, Arden N, Avouac B, Bruyère O. Safety of intra-articular hyaluronic acid injections in osteoarthritis: outcomes of
a systematic review and meta-analysis. Drugs Aging. 2019 Apr;36(Suppl 1):101-27. Also available:
http://dx.doi.org/10.1007/s40266-019-00657-w
. PMID: 31073925.
Material Performance Study - Hyaluronic Acid
|
167
14. Concoff A, Sancheti P, Niazi F, Shaw P, Rosen J. The efficacy of multiple versus single hyaluronic acid injections: a
systematic review and meta-analysis. BMC Musculoskelet Disord. 2017 Dec 21;18(1):542. Also available:
http://dx.doi.org/10.1186/s12891-017-1897-2
. PMID: 29268731.
15. O'Hanlon CE, Newberry SJ, Booth M, Grant S, Motala A, Maglione MA, FitzGerald JD, Shekelle PG. Hyaluronic acid
injection therapy for osteoarthritis of the knee: concordant efficacy and conflicting serious adverse events in two
systematic reviews. Syst Rev. 2016 Nov 4;5(1):186. Also available: http://dx.doi.org/10.1186/s13643-016-0363-9
.
PMID: 27814744.
16. Fang T, Li Q, Zhou F, Liu F, Liu Z, Zhao M, Chen M, You J, Jin Y, Xie J. Effect and safety of acupotomy in treatment of
knee osteoarthritis: a systematic review and meta-analysis. J Tradit Chin Med. 2020 Jun 15;40(3):355-64. PMID:
32506848.
17. Kim MS, Cho RK, In Y, Lai JH. The efficacy and safety of polydeoxyribonucleotide for the treatment of knee
osteoarthritis: systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2019
Sep;98(39):e17386. Also available: http://dx.doi.org/10.1097/MD.0000000000017386
. PMID: 31574892.
18. Park YB, Kim JH, Ha CW, Lee DH. Clinical efficacy of platelet-rich plasma injection and its association with growth
factors in the treatment of mild to moderate knee osteoarthritis: a randomized double-blind controlled clinical trial as
compared with hyaluronic acid. Am J Sports Med. 2021 Feb;49(2):487-96. Also available:
http://dx.doi.org/10.1177/0363546520986867
. PMID: 33523756.
19. Raeissadat SA, Ahangar AG, Rayegani SM, Sajjadi MM, Ebrahimpour A, Yavari P. Platelet-rich plasma-derived growth
factor vs hyaluronic acid injection in the individuals with knee osteoarthritis: a one year randomized clinical trial. J Pain
Res. 2020 Jul 8;13:1699-711. Also available: http://dx.doi.org/10.2147/JPR.S210715
. PMID: 32753945.
20. Ke Y, Jiang W, Xu Y, Chen Y, Zhang Q, Xue Q, Lin J, Ngai W, Nian G, Fazeli MS, Xie Y, Zhu Z. Efficacy and safety of a
single intra-articular injection of 6 ml Hylan G-F 20 compared to placebo in Chinese patients with symptomatic knee
osteoarthritis: C-SOUND study, a 26-week multicenter double-blind randomized placebo-controlled trial in China. BMC
Musculoskelet Disord. 2021 May 8;22(1):428. Also available: http://dx.doi.org/10.1186/s12891-021-04252-2
. PMID:
33964907.
21. Petterson SC, Plancher KD. Single intra-articular injection of lightly cross-linked hyaluronic acid reduces knee pain in
symptomatic knee osteoarthritis: a multicenter, double-blind, randomized, placebo-controlled trial. Knee Surg Sports
Traumatol Arthrosc. 2019 Jun;27(6):1992-2002. Also available: http://dx.doi.org/10.1007/s00167-018-5114-0
. PMID:
30159738.
22. Hangody L, Szody R, Lukasik P, Zgadzaj W, Lénárt E, Dokoupilova E, Bichovsk D, Berta A, Vasarhelyi G, Ficzere A,
Hangody G, Stevens G, Szendroi M. Intraarticular injection of a cross-linked sodium hyaluronate combined with
triamcinolone hexacetonide (Cingal) to provide symptomatic relief of osteoarthritis of the knee: a randomized, double-
blind, placebo-controlled multicenter clinical trial. Cartilage. 2018 Jul;9(3):276-83. Also available:
http://dx.doi.org/10.1177/1947603517703732
. PMID: 28535076.
23. Petrella RJ, Decaria J, Petrella MJ. Long term efficacy and safety of a combined low and high molecular weight
hyaluronic acid in the treatment of osteoarthritis of the knee. Rheumatol Rep. 2011;3(1):16-21.
24. Chen AF, Khalouf F, Zora K, Depalma M, Kohan L, Guirguis M, Beall D, Loudermilk E, Pingree MJ, Badiola I, Lyman J.
Cooled radiofrequency ablation provides extended clinical utility in the management of knee osteoarthritis: 12-month
results from a prospective, multi-center, randomized, cross-over trial comparing cooled radiofrequency ablation to a
single hyaluronic acid injection. BMC Musculoskelet Disord. 2021 May 8;21(1):428. Also available:
http://dx.doi.org/10.1186/s12891-020-03380-5
. PMID: 32517739.
25. Rezasoltani Z, Azizi S, Najafi S, Sanati E, Dadarkhah A, Abdorrazaghi F. Physical therapy, intra-articular dextrose
prolotherapy, botulinum neurotoxin, and hyaluronic acid for knee osteoarthritis: randomized clinical trial. Int J Rehabil
Res. 2020 Sep;43(3):219-27. Also available: http://dx.doi.org/10.1097/MRR.0000000000000411
. PMID: 32776763.
26. Hermans J, Bierma-Zeinstra SMA, Bos PK, Niesten DD, Verhaar JAN, Reijman M. The effectiveness of high molecular
weight hyaluronic acid for knee osteoarthritis in patients in the working age: a randomised controlled trial. BMC
Musculoskelet Disord. 2019 May 7;20(1):196. Also available: http://dx.doi.org/10.1186/s12891-019-2546-8
. PMID:
31064359.

Material Performance Study - Hyaluronic Acid
|
168
27. Saeed K, Khan SA, Ahmed I. Efficacy of intra articular hyaluronic acid versus arthroscopic debridement in terms of
improvement in pain score in Kellgran-Lawrence Grading II & III osteoarthritis of knee joint. Pak J Med Health Sci.
2015 Jul;9(3):1011-5.
28. Liao YY, Lin T, Zhu HX, Shi MM, Yan SG. Intra-articular viscosupplementation for patients with hip osteoarthritis: a
meta-analysis and systematic review. Med Sci Monit. 2019 Aug 27;25:6436-45. Also available:
http://dx.doi.org/10.12659/MSM.916955
. PMID: 31454342.
29. Leite VF, Daud Amadera JE, Buehler AM. Viscosupplementation for hip osteoarthritis: a systematic review and meta-
analysis of the efficacy on pain and disability, and the occurrence of adverse events. Arch Phys Med Rehabil. 2018
Mar;99(3):574-583.e1. Also available: http://dx.doi.org/10.1016/j.apmr.2017.07.010
. PMID: 28803906.
30. Ye Y, Zhou X, Mao S, Zhang J, Lin B. Platelet rich plasma versus hyaluronic acid in patients with hip osteoarthritis: a
meta-analysis of randomized controlled trials. Int J Surg. 2018 May;53:279-87. Also available:
http://dx.doi.org/10.1016/j.ijsu.2018.03.078
. PMID: 29626641.
31. Wu B, Li YM, Liu YC. Efficacy of intra-articular hyaluronic acid injections in hip osteoarthritis: a meta-analysis of
randomized controlled trials. Oncotarget. 2017 Sep 18;8(49):86865-76. Also available:
http://dx.doi.org/10.18632/oncotarget.20995. PMID: 29156841.
32. Boffa A, Previtali D, Di Laura Frattura G, Vannini F, Candrian C, Filardo G. Evidence on ankle injections for
osteochondral lesions and osteoarthritis: a systematic review and meta-analysis. Int Orthop. 2021 Feb;45(2):509-23.
Also available: http://dx.doi.org/10.1007/s00264-020-04689-5
. PMID: 32647968.
33. Kroon FPB, Rubio R, Schoones JW, Kloppenburg M. Intra-articular therapies in the treatment of hand osteoarthritis: a
systematic literature review. Drugs Aging. 2016 Feb;33(2):119-33. Also available:
http://dx.doi.org/10.1007/s40266-
015-0330-5. PMID: 26650235.
34. Zhang B, Thayaparan A, Horner N, Bedi A, Alolabi B, Khan M. Outcomes of hyaluronic acid injections for glenohumeral
osteoarthritis: a systematic review and meta-analysis. J Shoulder Elbow Surg. 2019 Mar;28(3):596-606. Also available:
http://dx.doi.org/10.1016/j.jse.2018.09.011
. PMID: 30502030.
35. Lee LC, Lieu FK, Lee HL, Tung TH. Effectiveness of hyaluronic acid administration in treating adhesive capsulitis of the
shoulder: a systematic review of randomized controlled trials. Biomed Res Int. 2015;2015:314120. Also available:
http://dx.doi.org/10.1155/2015/314120
. PMID: 25802845.
36. Liu Y, Wu J, Fei W, Cen X, Xiong Y, Wang S, Tang Y, Liang X. Is there a difference in intra-articular injections of
corticosteroids, hyaluronate, or placebo for temporomandibular osteoarthritis? J Oral Maxillofac Surg. 2018
Mar;76(3):504-14. Also available: http://dx.doi.org/10.1016/j.joms.2017.10.028
. PMID: 29182905.
37. Haigler MC, Abdulrehman E, Siddappa S, Kishore R, Padilla M, Enciso R. Use of platelet-rich plasma, platelet-rich growth
factor with arthrocentesis or arthroscopy to treat temporomandibular joint osteoarthritis: systematic review with meta-
analyses. J Am Dent Assoc. 2018 Nov;149(11):940-52. Also available: http://dx.doi.org/10.1016/j.adaj.2018.07.025.
PMID: 30724168.
38. Glasbrenner J, Petersen W, Raschke MJ, Steiger M, Verdonk R, Castelli CC, Zappalà G, Fritschy D, Herbort M. Matrix-
augmented bone marrow stimulation with a polyglycolic acid membrane with hyaluronan vs microfracture in local
cartilage defects of the femoral condyles: a multicenter randomized controlled trial. Orthop J Sports Med. 2020 May
29;8(5):2325967120922938. Also available: http://dx.doi.org/10.1177/2325967120922938
. PMID: 32528994.
39. Sofu H, Camurcu Y, Ucpunar H, Ozcan S, Yurten H, Sahin V. Clinical and radiographic outcomes of chitosan-glycerol
phosphate/blood implant are similar with hyaluronic acid-based cell-free scaffold in the treatment of focal
osteochondral lesions of the knee joint. Knee Surg Sports Traumatol Arthrosc. 2019 Mar;27(3):773-81. Also available:
http://dx.doi.org/10.1007/s00167-018-5079-z
. PMID: 30069652.
40. Sofu H, Kockara N, Oner A, Camurcu Y, Issn A, Sahin V. Results of hyaluronic acid-based cell-free scaffold application in
combination with microfracture for the treatment of osteochondral lesions of the knee: 2-year comparative study.
Arthroscopy. 2017 Jan;33(1):209-16. Also available: http://dx.doi.org/10.1016/j.arthro.2016.06.035
. PMID: 27614391.
41. Gorbea E, Kidwai S, Rosenberg J. Nonsurgical tear trough volumization: a systematic review of patient satisfaction.
Aesthet Surg J. 2021 Jul 14;41(8):NP1053-NP1060. Also available: http://dx.doi.org/10.1093/asj/sjab116
. PMID:
33693530.

Material Performance Study - Hyaluronic Acid
|
169
42. Kumar V, Jain A, Atre S, Shome D, Kapoor R, Doshi K, Vadera S. Non-surgical rhinoplasty using hyaluronic acid dermal
fillers: a systematic review. J Cosmet Dermatol. 2021 Aug;20(8):2414-24. Also available:
http://dx.doi.org/10.1111/jocd.14173
. PMID: 33900020.
43. Stefura T, Kacprzyk A, Dros J, Krzysztofik M, Skomarovska O, Fijalkowska M, Koziej M. Tissue fillers for the nasolabial
fold area: a systematic review and meta-analysis of randomized clinical trials. Aesthetic Plast Surg. 2021
Oct;45(5):2300-16. Also available: http://dx.doi.org/10.1007/s00266-021-02439-5
. PMID: 34255156.
44. Chung KL, Convery C, Ejikeme I, Ghanem AM. A systematic review of the literature of delayed inflammatory reactions
after hyaluronic acid filler injection to estimate the incidence of delayed type hypersensitivity reaction. Aesthet Surg J.
2020 Apr 14;40(5):NP286-NP300. Also available: http://dx.doi.org/10.1093/asj/sjz222
. PMID: 31410442.
45. Kapoor KM, Kapoor P, Heydenrych I, Bertossi D. Vision loss associated with hyaluronic acid fillers: a systematic review
of literature. Aesthetic Plast Surg. 2020 Jun;44(3):929-44. Also available:
http://dx.doi.org/10.1007/s00266-019-01562-
8. PMID: 31822960.
46. Stojanovi L, Majdi N. Effectiveness and safety of hyaluronic acid fillers used to enhance overall lip fullness: a systematic
review of clinical studies. J Cosmet Dermatol. 2019 Apr;18(2):436-43. Also available:
http://dx.doi.org/10.1111/jocd.12861. PMID: 30636365.
47. Beer K, Kaufman-Janette J, Bank D, Biesman B, Dayan S, Kim W, Chawla S, Schumacher A. Safe and effective chin
augmentation with the hyaluronic acid injectable filler, VYC-20L. Dermatol Surg. 2021 Jan;47(1):80-5. Also available:
http://dx.doi.org/10.1097/DSS.0000000000002795
. PMID: 33347003.
48. Cheon HI, Kim JH, Kim BJ, Lee YW. Efficacy and safety of a new hyaluronic acid filler for nasolabial folds: a 52-week,
multicenter, randomized, evaluator/subject-blind, split-face study. J Cosmet Dermatol. 2021 May;20(5):1467-73. Also
available: http://dx.doi.org/10.1111/jocd.13773
. PMID: 33085170.
49. Ogilvie P, Benouaiche L, Philipp-Dormston WG, Belhaouari L, Gaymans F, Sattler G, Harvey C, Schumacher A. VYC-25L
hyaluronic acid injectable gel is safe and effective for long-term restoration and creation of volume of the lower face.
Aesthet Surg J. 2020 Aug 14;40(9):NP499-NP510. Also available: http://dx.doi.org/10.1093/asj/sjaa013
. PMID:
31960896.
50. Dai X, Li L, Peterson W, Baumgartner RR, Huang J, Baer-Zwick A, Hoeller S, Ivezic-Schoenfeld Z, Prinz M. Safety and
effectiveness of hyaluronic acid dermal filler in correction of moderate-to-severe nasolabial folds in Chinese subjects.
Clin Cosmet Investig Dermatol. 2019 Jan 14;12:57-62. Also available: http://dx.doi.org/10.2147/CCID.S187079
. PMID:
30666143.
51. Kaufman-Janette J, Taylor SC, Cox SE, Weinkle SH, Smith S, Kinney BM. Efficacy and safety of a new resilient
hyaluronic acid dermal filler, in the correction of moderate-to-severe nasolabial folds: a 64-week, prospective,
multicenter, controlled, randomized, double-blind and within-subject study. J Cosmet Dermatol. 2019 Oct;18(5):1244-
53. Also available: http://dx.doi.org/10.1111/jocd.13100
. PMID: 31444861.
52. Li D, Sun J, Wu S. A multi-center comparative efficacy and safety study of two different hyaluronic acid fillers for
treatment of nasolabial folds in a Chinese population. J Cosmet Dermatol. 2019 Jun;18(3):755-61. Also available:
http://dx.doi.org/10.1111/jocd.12916
. PMID: 31074161.
53. Wu Y, Tian Y, Xu J, Zhong S, Wang R, Wu W. A randomized study showing improved skin quality and aesthetic
appearance of dorsal hands after hyaluronic acid gel treatment in a Chinese population. J Cosmet Dermatol. 2020
Jul;19(7):1627-35. Also available: http://dx.doi.org/10.1111/jocd.13221
. PMID: 31769596.
54. Prager W, Agsten K, Kravtsov M, Kerscher M. Mid-face volumization with hyaluronic acid: injection technique and safety
aspects from a controlled, randomized, double-blind clinical study. J Drugs Dermatol. 2017 Apr 1;16(4):351-7. PMID:
28403269.
55. Weiss RA, Moradi A, Bank D, Few J, Joseph J, Dover J, Lin X, Nogueira A, Mashburn J. Effectiveness and safety of large
gel particle hyaluronic acid with lidocaine for correction of midface volume deficit or contour deficiency. [Erratum
appears in Dermatol Surg. 2016 Oct;42(10):1233]. Dermatol Surg. 2016 Jun;42(6):699-709. Also available:
http://dx.doi.org/10.1097/DSS.0000000000000771
. PMID: 27176869.
56. Butterwick K, Marmur E, Narurkar V, Cox SE, Joseph JH, Sadick NS, Tedaldi R, Wheeler S, Kolodziejczyk JK, Gallagher
CJ. HYC-24L demonstrates greater effectiveness with less pain than CPM-22.5 for treatment of perioral lines in a
randomized controlled trial. Dermatol Surg. 2015;41(12):1351-60. Also available:
http://dx.doi.org/10.1097/DSS.0000000000000576
. PMID: 26606435.

Material Performance Study - Hyaluronic Acid
|
170
57. Sattler G, Walker T, Buxmeyer B, Biwer B. Efficacy of calcium hydroxylapatite filler versus hyaluronic acid filler in hand
augmentation. Aktuel Dermatol. 2014 Nov;40(11):445-51. Also available: http://dx.doi.org/10.1055/s-0034-1378110
.
58. Wortsman X, Moll-Manzur C, Ramírez-Cornejo C, Alfaro-Sepúlveda D, Mellado-Francisco G, Rezende J, Vera-Kellet C.
Ultrasonographic subclinical signs of inflammation of the lacrimal, parotid, and submandibular glands in users of
cosmetic fillers. J Ultrasound Med. 2021 Nov;40(11):2377-89. Also available: http://dx.doi.org/10.1002/jum.15621
.
PMID: 33417303.
59. Sadeghpour M, Quatrano NA, Bonati LM, Arndt KA, Dover JS, Kaminer MS. Delayed-onset nodules to differentially
crosslinked hyaluronic acids: comparative incidence and risk assessment. Dermatol Surg. 2019 Aug;45(8):1085-94. Also
available: http://dx.doi.org/10.1097/DSS.0000000000001814
. PMID: 30789508.
60. Ueland HO, Nilsen RM, Rødahl E, Jensen SA. Hyaluronic acid is superior to autologous fat for treatment of temporal
hollowing after lateral orbital wall decompression: a prospective interventional trial. J Plast Reconstr Aesthet Surg. 2019
Jun;72(6):973-81. Also available: http://dx.doi.org/10.1016/j.bjps.2018.12.031
. PMID: 30679116.
61. Sito G, Marlino S, Santorelli A. Use of Macrolane VRF 30 in emicircumferential penis enlargement. Aesthetic Surg J.
2013 Feb;33(2):258-64. Also available: http://dx.doi.org/10.1177/1090820X12472337
. PMID: 23388646.
62. Lee KE, Kim GJ, Sa HS. The clinical spectrum of periorbital vascular complications after facial injection. J Cosmet
Dermatol. 2021 May;20(5):1532-40. Also available: http://dx.doi.org/10.1111/jocd.14019
. PMID: 33615645.
63. Munavalli GG, Guthridge R, Knutsen-Larson S, Brodsky A, Matthew E, Landau M. "COVID-19/SARS-CoV-2 virus spike
protein-related delayed inflammatory reaction to hyaluronic acid dermal fillers: a challenging clinical conundrum in
diagnosis and treatment?" Arch Dermatol Res. 2021 Feb 9;Online ahead of print. Also available:
http://dx.doi.org/10.1007/s00403-021-02190-6
. PMID: 33559733.
64. Humphrey S, Jones DH, Carruthers JD, Carruthers A, Beleznay K, Wesley N, Black JM, Vanderveen S, Minokadeh A.
Retrospective review of delayed adverse events secondary to treatment with a smooth, cohesive 20-mg/mL hyaluronic
acid filler in 4500 patients. J Am Acad Dermatol. 2020 Jul 1;83(1):86-95. Also available:
http://dx.doi.org/10.1016/j.jaad.2020.01.066
. PMID: 32035107.
65. Kassir R, Venkataram A, Malek A, Rao D. Non-surgical rhinoplasty: the ascending technique and a 14-year retrospective
study of 2130 cases. Aesthetic Plast Surg. 2021 Jun;45(3):1154-68. Also available:
http://dx.doi.org/10.1007/s00266-
020-02048-8. PMID: 33216177.
66. Zhang FF, Xu ZX, Chen Y. Delayed foreign body granulomas in the orofacial region after hyaluronic acid injection. Chin
J Dent Res. 2020;23(4):289-96. Also available: http://dx.doi.org/10.3290/j.cjdr.b867893
. PMID: 33491361.
67. Turkmani MG, Boulle KD, Philipp-Dormston WG. Delayed hypersensitivity reaction to hyaluronic acid dermal filler
following influenza-like illness. Clin Cosmet Investig Dermatol. 2019 Apr 29;12:277-83. Also available:
http://dx.doi.org/10.2147/CCID.S198081
.
68. Beleznay K, Carruthers JDA, Carruthers A, Mummert ME, Humphrey S. Delayed-onset nodules secondary to a smooth
cohesive 20 mg/mL hyaluronic acid filler: cause and management. Dermatol Surg. 2015 Aug 6;41(8):929-39. Also
available: http://dx.doi.org/10.1097/DSS.0000000000000418
. PMID: 26166260.
69. Trignano E, Rusciani A, Armenti AF, Corrias F, Fallico N. Augmentation mammaplasty after breast enhancement with
hyaluronic acid. Aesthet Surg J. 2015 Aug;35(6):NP161-8. Also available: http://dx.doi.org/10.1093/asj/sjv042
. PMID:
25911630.
70. Inglefield C. Early clinical experience of hyaluronic acid gel for breast enhancement. J Plast Reconstr Aesthet Surg.
2011 Jun;64(6):722-9. Also available: http://dx.doi.org/10.1016/j.bjps.2010.09.015
. PMID: 20951657.
71. Park TH, Seo SW, Kim JK, Chang CH. Clinical experience with hyaluronic acid-filler complications. J Plast Reconstr
Aesthet Surg. 2011 Jul;64(7):892-7. Also available: http://dx.doi.org/10.1016/j.bjps.2011.01.008
. PMID: 21310674.
72. Alijotas-Reig J, Esteve-Valverde E, Gil-Aliberas N, Garcia-Gimenez V. Autoimmune/inflammatory syndrome induced by
adjuvants-ASIA-related to biomaterials: analysis of 45 cases and comprehensive review of the literature. Immunol Res.
2018 Feb;66(1):120-40. Also available: http://dx.doi.org/10.1007/s12026-017-8980-5
. PMID: 29199390.
73. Alijotas-Reig J, Garcia-Gimenez V, Llurba E, Vilardell-Tarrés M. Autoimmune/inflammatory syndrome (ASIA) induced by
biomaterials injection other than silicone medical grade. Lupus. 2012 Oct;21(12):1326-34. Also available:
http://dx.doi.org/10.1177/0961203312458838
. PMID: 22952322.

Material Performance Study - Hyaluronic Acid
|
171
74. Nie F, Xie H, Wang G, An Y. Risk comparison of filler embolism between polymethyl methacrylate (PMMA) and
hyaluronic acid (HA). Aesthetic Plast Surg. 2019 Jun;43(3):853-60. Also available:
http://dx.doi.org/10.1007/s00266-
019-01320-w. PMID: 30824948.
75. Malvankar-Mehta MS, Fu A, Subramanian Y, Hutnik C. Impact of ophthalmic viscosurgical devices in cataract surgery. J
Ophthalmol. 2020 Oct 20;2020:7801093. Also available: http://dx.doi.org/10.1155/2020/7801093
. PMID: 33133677.
76. Auffarth GU, Auerbach FN, Rabsilber T, Gegúndez JA, Cuiña R, Renard Y, Vinciguerra P, Camesasca F, Van
Cauwenberge F, Amzallag T, Van Setten G, Holzer MP. Comparison of the performance and safety of 2 ophthalmic
viscosurgical devices in cataract surgery. J Cataract Refract Surg. 2017 Jan;43(1):87-94. Also available:
http://dx.doi.org/10.1016/j.jcrs.2016.10.025
. PMID: 28317684.
77. Modi SS, Davison JA, Walters T. Safety, efficacy, and intraoperative characteristics of DisCoVisc and Healon ophthalmic
viscosurgical devices for cataract surgery. Clin Ophthalmol. 2011;5(1):1381-9. Also available:
http://dx.doi.org/10.2147/opth.s22243
.
78. Dugan C, Zheng CX, Lin MM, Ustaoglu M, Zhang Q, Hamershock RA, Moster SJ, Moster MR. Outcomes of Ahmed
glaucoma valve implantation with viscoelastic fill at both beginning and end of surgery. J Glaucoma. 2020
Aug;29(8):704-10. Also available: http://dx.doi.org/10.1097/IJG.0000000000001536. PMID: 32398592.
79. Uemoto R, Nakasato-Sonn H, Meguro A, Ito N, Yazama F, Mizuki N. Staining internal limiting membrane with a mixture
of BBG and sodium hyaluronate. Br J Ophthalmol. 2013 Jun;97(6):690-3. Also available:
http://dx.doi.org/10.1136/bjophthalmol-2012-302289
. PMID: 23203697.
80. Leszczyski R, Formiska-Kapucik M, Bubaa-Stachowicz B, Mrukwa-Kominek E, Filipek E, Pawlicki K. Nonpenetrating very
deep sclerectomy with hyaluronic acid implant vs trabeculectomy - a 2-year follow-up. Graefes Arch Clin Exp
Ophthalmol. 2012 Dec;250(12):1835-41. Also available: http://dx.doi.org/10.1007/s00417-012-1985-9
. PMID:
22569857.
81. Fezza JP. Cross-linked hyaluronic acid gel occlusive device for the treatment of dry eye syndrome. Clin Ophthalmol.
2018 Nov 8;12:2277-83. Also available: http://dx.doi.org/10.2147/OPTH.S187963
. PMID: 30510396.
82. Altintas A, Ciritolu M, Beyazyildiz O, Can C, Polat S. Toxic anterior segment syndrome outbreak after cataract surgery
triggered by viscoelastic substance. Middle East Afr J Ophthalmol. 2017 Jan;24(1):43-7. Also available:
http://dx.doi.org/10.4103/meajo.MEAJO_226_15
. PMID: 28546691.
83. Jaramillo A, Foreman J, Ayyala RS. Descemet membrane detachment after canaloplasty: incidence and management. J
Glaucoma. 2014 Aug;23(6):351-54. Also available: http://dx.doi.org/10.1097/IJG.0b013e318279ca7f
. PMID: 23221908.
84. Shafi F, Agrawal P, Holder R, Sung V. Bleb needling with subconjunctival injection of sodium hyaluronate 1.4%: 1-year
outcomes. Can J Ophthalmol. 2011 Dec;46(6):537-42. Also available: http://dx.doi.org/10.1016/j.jcjo.2011.09.005
.
PMID: 22153643.
85. Groß D, Childs M, Piaton JM. Comparative study of 0.1% hyaluronic acid versus 0.5% carboxymethylcellulose in
patients with dry eye associated with moderate keratitis or keratoconjunctivitis. Clin Ophthalmol. 2018 Jun 11;12:1081-
8. Also available: http://dx.doi.org/10.2147/OPTH.S161578
. PMID: 29928109.
86. Cagini C, Torroni G, Fiore T, Cerquaglia A, Lupidi M, Aragona P, Iaccheri B. Tear film stability in Sjögren syndrome
patients treated with hyaluronic acid versus crosslinked hyaluronic acid-based eye drops. J Ocul Pharmacol Ther. 2017
Sep 1;33(7):539-42. Also available: http://dx.doi.org/10.1089/jop.2016.0149
. PMID: 28557655.
87. Groß D, Childs M, Piaton JM. Comparison of 0.2% and 0.18% hyaluronate eye drops in patients with moderate to
severe dry eye with keratitis or keratoconjunctivitis. Clin Ophthalmol. 2017 Apr 6;11:631-8. Also available:
http://dx.doi.org/10.2147/OPTH.S131384
. PMID: 28435213.
88. Lambiase A, Sullivan BD, Schmidt TA, Sullivan DA, Jay GD, Truitt ER, Bruscolini A, Sacchetti M, Mantelli F. A two-week,
randomized, double-masked study to evaluate safety and efficacy of lubricin (150 µg/mL) eye drops versus sodium
hyaluronate (HA) 0.18% eye drops (Vismed®) in patients with moderate dry eye disease. Ocul Surf. 2017
Jan;15(1):77-87. Also available: http://dx.doi.org/10.1016/j.jtos.2016.08.004
. PMID: 27614318.
89. Gong L, Sun X, Ma Z, Wang Q, Xu X, Chen X, Shao Y, Yao K, Tang L, Gu Y, Yuan H, Chua WH, Chuan JCY, Tong L. A
randomised, parallel-group comparison study of diquafosol ophthalmic solution in patients with dry eye in China and
Singapore. Br J Ophthalmol. 2015 Jul;99(7):903-8. Also available:
http://dx.doi.org/10.1136/bjophthalmol-2014-
306084. PMID: 25631485.

Material Performance Study - Hyaluronic Acid
|
172
90. Prabhasawat P, Ruangvaravate N, Tesavibul N, Thewthong M. Effect of 0.3% hydroxypropyl methylcellulose/dextran
versus 0.18% sodium hyaluronate in the treatment of ocular surface disease in glaucoma patients: a randomized,
double-blind, and controlled study. J Ocul Pharmacol Ther. 2015 Jul;31(6):323-9. Also available:
http://dx.doi.org/10.1089/jop.2014.0115
. PMID: 26090941.
91. Jee D, Park SH, Kim MS, Kim EC. Antioxidant and inflammatory cytokine in tears of patients with dry eye syndrome
treated with preservative-free versus preserved eye drops. Invest Ophthalmol Vis Sci. 2014 Jul 3;55(8):5081-9. Also
available: http://dx.doi.org/10.1167/iovs.14-14483
. PMID: 24994869.
92. Liu X, Wang S, Kao AA, Long Q. The effect of topical pranoprofen 0.1% on the clinical evaluation and conjunctival HLA-
DR expression in dry eyes. Cornea. 2012 Nov;31(11):1235-9. Also available:
http://dx.doi.org/10.1097/ICO.0b013e31824988e5
. PMID: 22677643.
93. Labetoulle M, Chiambaretta F, Shirlaw A, Leaback R, Baudouin C. Osmoprotectants, carboxymethylcellulose and
hyaluronic acid multi-ingredient eye drop: a randomised controlled trial in moderate to severe dry eye. Eye. 2017
Oct;31(10):1409-16. Also available: http://dx.doi.org/10.1038/eye.2017.73
. PMID: 28452989.
94. Chiambaretta F, Doan S, Labetoulle M, Rocher N, El Fekih L, Messaoud R, Khairallah M, Baudouin C. A randomized,
controlled study of the efficacy and safety of a new eyedrop formulation for moderate to severe dry eye syndrome. Eur
J Ophthalmol. 2017 Jan;27(1):1-9. Also available: http://dx.doi.org/10.5301/ejo.5000836. PMID: 27445067.
95. Robert PY, Cochener B, Amrane M, Ismail D, Garrigue JS, Pisella PJ, Baudouin C. Efficacy and safety of a cationic
emulsion in the treatment of moderate to severe dry eye disease: a randomized controlled study. Eur J Ophthalmol.
2016 Nov;26(6):546-55. Also available: http://dx.doi.org/10.5301/ejo.5000830
. PMID: 27515572.
96. Kinoshita S, Oshiden K, Awamura S, Suzuki H, Nakamichi N, Yokoi N. A randomized, multicenter phase 3 study
comparing 2% rebamipide (OPC-12759) with 0.1% sodium hyaluronate in the treatment of dry eye. Ophthalmology.
2013 Jun;120(6):1158-65. Also available: http://dx.doi.org/10.1016/j.ophtha.2012.12.022
. PMID: 23490326.
97. Takamura E, Tsubota K, Watanabe H, Ohashi Y. A randomised, double-masked comparison study of diquafosol versus
sodium hyaluronate ophthalmic solutions in dry eye patients. Br J Ophthalmol. 2012 Oct;96(10):1310-5. Also available:
http://dx.doi.org/10.1136/bjophthalmol-2011-301448
. PMID: 22914501.
98. Baudouin C, Cochener B, Pisella PJ, Girard B, Pouliquen P, Cooper H, Creuzot-Garcher C. Randomized, phase III study
comparing osmoprotective carboxymethylcellulose with sodium hyaluronate in dry eye disease. Eur J Ophthalmol. 2012
Sep-Oct;22(5):751-61. Also available: http://dx.doi.org/10.5301/ejo.5000117
. PMID: 22287172.
99. Casale M, Moffa A, Vella P, Sabatino L, Capuano F, Salvinelli B, Lopez MA, Carinci F, Salvinelli F. Hyaluronic acid:
perspectives in dentistry. A systematic review. Int J Immunopathol Pharmacol. 2016;29(4):572-82. Also available:
http://dx.doi.org/10.1177/0394632016652906
. PMID: 27280412.
100. Chandel AKS, Shimizu A, Hasegawa K, Ito T. Advancement of biomaterial-based postoperative adhesion barriers.
Macromol Biosci. 2021 Mar;21(3):e2000395. Also available: http://dx.doi.org/10.1002/mabi.202000395
. PMID:
33463888.
101. Redorta JP, Sanguedolce F, Pardo GS, Romancik M, Vittori G, Minervini A, Di Maida F, Lunik R, Colombo R, Serretta V,
Cetinel B, Bini V, Corradengo D, Lazzeri M. Multicentre International Study for the Prevention with iAluRil of Radio-
induced Cystitis (MISTIC): a randomised controlled study. Eur Urol Open Sci. 2021 Feb 23;26:45-54. Also available:
http://dx.doi.org/10.1016/j.euros.2021.01.016
. PMID: 34337507.
102. Hajibandeh S, Hajibandeh S, Saeed S, Bird J, Kannappa L, Ratnayake I. Effect of hyaluronate-based bioresorbable
membrane (Seprafilm) on outcomes of abdominal surgery: a meta-analysis and trial sequential analysis of randomised
controlled trials. Updates Surg. 2021 Jun 20;Online ahead of print. Also available:
http://dx.doi.org/10.1007/s13304-
021-01117-0. PMID: 34148173.
103. Guo Y, Zhu Q, Chen S, Li Y, Fu D, Qiao D, Wang Y, Yang Y. Effect of sodium hyaluronate-arboxycellulose membrane
(Seprafilm®) on postoperative small bowel obstruction: a meta-analysis. Surgery. 2021 Jun;169(6):1333-9. Also
available: http://dx.doi.org/10.1016/j.surg.2020.12.004
. PMID: 33461779.
104. Zhao J, Huang C, Zhu J, Zhu J, Yuan R, Zhu Z. Efficacy and safety of Seprafilm for preventing intestinal obstruction
after gastrointestinal neoplasms surgery: a systematic review and meta-analysis. Acta Chir Belg. 2021 Feb;121(1):1-15.
Also available: http://dx.doi.org/10.1080/00015458.2020.1871286
. PMID: 33459577.
Material Performance Study - Hyaluronic Acid
|
173
105. Ouaissi M, Gaujoux S, Veyrie N, Denève E, Brigand C, Castel B, Duron JJ, Rault A, Slim K, Nocca D. Post-operative
adhesions after digestive surgery: their incidence and prevention: review of the literature. J Visc Surg. 2012
Apr;149(2):e104-114. PMID: 22261580.
106. Dupré A, Lefranc A, Buc E, Delpero JR, Quenet F, Passot G, Evrard S, Rivoire M. Use of bioresorbable membranes to
reduce abdominal and perihepatic adhesions in 2-stage hepatectomy of liver metastases from colorectal cancer: results
of a prospective, randomized controlled phase II trial. Ann Surg. 2013 Jul;258(1):30-6. Also available:
http://dx.doi.org/10.1097/SLA.0b013e3182854949
. PMID: 23426344.
107. Saito G, Sadahiro S, Ogimi T, Miyakita H, Okada K, Tanaka A, Suzuki T. Preventive effects of a synthetic absorbable
antiadhesive film (seprafilm) on small bowel obstruction in patients who underwent elective surgery for colon cancer: a
randomized controlled trial. J Surg Oncol. 2019 Nov;120(6):1038-43. Also available:
http://dx.doi.org/10.1002/jso.25664
. PMID: 31392725.
108. Kiefer DG, Muscat JC, Santorelli J, Chavez MR, Ananth CV, Smulian JC, Vintzileos AM. Effectiveness and short-term
safety of modified sodium hyaluronic acid-carboxymethylcellulose at cesarean delivery: a randomized trial presented at
the Annual Clinical Meetings of the American Congress of Obstetricians and Gynecologists, San Diego, CA, May 5-9,
2012 (safety data) and Chicago, IL, April 26-30, 2014 (primary outcome). Am J Obstet Gynecol. 2016
Mar;214(3):373.e1-373.e12. Also available: http://dx.doi.org/10.1016/j.ajog.2015.10.012
. PMID: 26478104.
109. Lee WK, Park YH, Choi S, Lee WS. Is liquid-based hyaluronic acid equivalent to sodium hyaluronate-based
bioresorbable membrane to reduce small bowel obstruction in patients undergoing colorectal surgery. Asian J Surg.
2019 Feb;42(2):443-9. Also available: http://dx.doi.org/10.1016/j.asjsur.2018.09.009
. PMID: 30527555.
110. Berdah SV, Mariette C, Denet C, Panis Y, Laurent C, Cotte E, Huten N, Le Peillet Feuillet E, Duron JJ. A multicentre,
randomised, controlled trial to assess the safety, ease of use, and reliability of hyaluronic acid/carboxymethylcellulose
powder adhesion barrier versus no barrier in colorectal laparoscopic surgery. Trials. 2014 Oct 27;15:413. Also available:
http://dx.doi.org/10.1186/1745-6215-15-413
. PMID: 25348087.
111. Fossum GT, Silverberg KM, Miller CE, Diamond MP, Holmdahl L. Gynecologic use of Sepraspray adhesion barrier for
reduction of adhesion development after laparoscopic myomectomy: a pilot study. Fertil Steril. 2011 Aug;96(2):487-91.
Also available: http://dx.doi.org/10.1016/j.fertnstert.2011.05.081
. PMID: 21718999.
112. Kim TN, Chung MK, Nam JK, Lee JZ, Chung JH, Lee SW. Effectiveness of hyaluronic acid/carboxymethylcellulose in
preventing adhesive bowel obstruction after laparoscopic radical cystectomy. Asian J Surg. 2019 Jan;42(1):394-400.
Also available: http://dx.doi.org/10.1016/j.asjsur.2018.08.007
. PMID: 30266466.
113. Chung JH, Moon HS, Choi HY, Jeong TY, Ha US, Han JH, Cho JM, Kim TH, Lee SW. Inhibition of adhesion and fibrosis
improves the outcome of epididymectomy as a treatment for chronic epididymitis: a multicenter, randomized controlled,
single-blind study. J Urol. 2013 May;189(5):1730-4. Also available: http://dx.doi.org/10.1016/j.juro.2012.11.168
. PMID:
23219538.
114. Mao X, Tao Y, Cai R, Zhang J, Gao H, Chen Q, Kuang Y, Zhang S. Cross-linked hyaluronan gel to improve pregnancy
rate of women patients with moderate to severe intrauterine adhesion treated with IVF: a randomized controlled trial.
Arch Gynecol Obstet. 2020 Jan;301(1):199-205. Also available: http://dx.doi.org/10.1007/s00404-019-05368-6
. PMID:
31883044.
115. Zhou Q, Shi X, Saravelos S, Huang X, Zhao Y, Huang R, Xia E, Li TC. Auto-cross-linked hyaluronic acid gel for
prevention of intrauterine adhesions after hysteroscopic adhesiolysis: a randomized controlled trial. J Minim Invasive
Gynecol. 2021 Feb;28(2):307-13. Also available: http://dx.doi.org/10.1016/j.jmig.2020.06.030
. PMID: 32681996.
116. Li X, Wu L, Zhou Y, Fan X, Huang J, Wu J, Yu R, Lou J, Yang M, Yao Z, Xue M. New crosslinked hyaluronan gel for the
prevention of intrauterine adhesions after dilation and curettage in patients with delayed miscarriage: a prospective,
multicenter, randomized, controlled trial. J Minim Invasive Gynecol. 2019 Jan;26(1):94-9. Also available:
http://dx.doi.org/10.1016/j.jmig.2018.03.032
. PMID: 29678756.
117. Can S, Kirpinar G, Dural O, Karamustafaoglu BB, Tas IS, Yasa C, Ugurlucan FG. Efficacy of a new crosslinked
hyaluronan gel in the prevention of intrauterine adhesions. JSLS. 2018 Oct-Dec;22(4):e2018.00036. Also available:
http://dx.doi.org/10.4293/JSLS.2018.00036
. PMID: 30524185.

Material Performance Study - Hyaluronic Acid
|
174
118. Liu C, Lu Q, Zhang Z, Xue M, Zhang Y, Zhang Y, Wang H, Li H, Zhou Y, Zhang Z, Li W, Zhai Y, Jiang Y, Sang C, Xiao S,
Xiao F, Ye M, Zhang A, Jiang J, Wang G, Yang X, Cui B, Lu Q, Meng Q, Zhang Q, Lu Y, Wang Y. A randomized
controlled trial on the efficacy and safety of a new crosslinked hyaluronan gel in reducing adhesions after gynecologic
laparoscopic surgeries. J Minim Invasive Gynecol. 2015;22(5):853-63. Also available:
http://dx.doi.org/10.1016/j.jmig.2015.04.011
. PMID: 25906706.
119. Mais V, Cirronis MG, Peiretti M, Ferrucci G, Cossu E, Melis GB. Efficacy of auto-crosslinked hyaluronan gel for adhesion
prevention in laparoscopy and hysteroscopy: a systematic review and meta-analysis of randomized controlled trials. Eur
J Obstet Gynecol Reprod Biol. 2012 Jan;160(1):1-5. Also available: http://dx.doi.org/10.1016/j.ejogrb.2011.08.002
.
PMID: 21945572.
120. Borghese G, Raffone A, Raimondo D, Saccone G, Travaglino A, Degli Esposti E, Mastronardi M, Salucci P, Zullo F,
Seracchioli R. Adhesion barriers in laparoscopic myomectomy: evidence from randomized clinical trials. Int J Gynecol
Obstet. 2021 Mar;152(3):308-20. Also available: http://dx.doi.org/10.1002/ijgo.13495
. PMID: 33237574.
121. Yan Y, Xu D. The effect of adjuvant treatment to prevent and treat intrauterine adhesions: a network meta-analysis of
randomized controlled trials. J Minim Invasive Gynecol. 2018 May;25(4):589-99. Also available:
http://dx.doi.org/10.1016/j.jmig.2017.09.006. PMID: 28893657.
122. Healy MW, Schexnayder B, Connell MT, Terry N, DeCherney AH, Csokmay JM, Yauger BJ, Hill MJ. Intrauterine adhesion
prevention after hysteroscopy: a systematic review and meta-analysis. Am J Obstet Gynecol. 2016 Sep;215(3):267-
275.e7. Also available: http://dx.doi.org/10.1016/j.ajog.2016.05.001
. PMID: 27173082.
123. Hindocha A, Beere L, Dias S, Watson A, Ahmad G. Adhesion prevention agents for gynaecological surgery: an overview
of Cochrane reviews. Cochrane Database Syst Rev. 2015 Jan 6;(1):CD011254. Also available:
http://dx.doi.org/10.1002/14651858.CD011254.pub2
. PMID: 25561409.
124. Fei Z, Xin X, Fei H, Yuechong C. Meta-analysis of the use of hyaluronic acid gel to prevent intrauterine adhesions after
miscarriage. Eur J Obstet Gynecol Reprod Biol. 2020 Jan;244:1-4. Also available:
http://dx.doi.org/10.1016/j.ejogrb.2019.10.018
. PMID: 31731019.
125. Zheng F, Xin X, He F, Liu J, Cui Y. Meta-analysis on the use of hyaluronic acid gel to prevent intrauterine adhesion after
intrauterine operations. Exp Ther Med. 2020;19(4):2672-8. Also available: http://dx.doi.org/10.3892/etm.2020.8483
.
126. Fei Z, Bin Z, Xin X, Fei H, Yuechong C. Meta-analysis on the use of hyaluronic acid gel to prevent recurrence of
intrauterine adhesion after hysteroscopic adhesiolysis. Taiwan J Obstet Gynecol. 2019 Nov;58(6):731-6. Also available:
http://dx.doi.org/10.1016/j.tjog.2019.09.002
. PMID: 31759520.
127. Liu H, Xu Y, Yi N, Yi W. Efficacy and safety of hyaluronic acid gel for the prevention of intrauterine adhesion: a meta-
analysis of randomized clinical trials. Gynecol Obstet Invest. 2018 May;83(3):227-33. Also available:
http://dx.doi.org/10.1159/000486674
. PMID: 29514160.
128. Tafti SZG, Javaheri A, Firoozabadi RD, Ashkezar SK, Abarghouei HF. Role of hyaluronic acid intrauterine injection in the
prevention of Asherman's syndrome in women undergoing uterine septum resection: an RCT. Int J Reprod Biomed
(Yazd). 2021;19(4):339-46. Also available: http://dx.doi.org/10.18502/ijrm.v19i4.9060
. PMID: 33997593.
129. Hooker AB, de Leeuw RA, van de Ven PM, Brölmann HAM, Huirne JAF. Reproductive performance after the application
of hyaluronic acid gel after dilation and curettage in women who have experienced at least one previous curettage:
long-term results of a multicenter prospective randomized trial. Fertil Steril. 2018 Dec;110(7):1231-8. Also available:
http://dx.doi.org/10.1016/j.fertnstert.2018.07.021
. PMID: 30503111.
130. Hooker AB, de Leeuw R, van de Ven PM, Bakkum EA, Thurkow AL, Vogel NEA, van Vliet HAAM, Bongers MY, Emanuel
MH, Verdonkschot AEM, Brölmann HAM, Huirne JAF. Prevalence of intrauterine adhesions after the application of
hyaluronic acid gel after dilatation and curettage in women with at least one previous curettage: short-term outcomes
of a multicenter, prospective randomized controlled trial. Fertil Steril. 2017 May;107(5):1223-1231.e3. Also available:
http://dx.doi.org/10.1016/j.fertnstert.2017.02.113
. PMID: 28390688.
131. Ekin M, Kaya C, Erdogan V, Bahceci E, Baghaki S, Yasar L. The effect of new cross linked hyaluronan gel on quality of
life of patients after deep infiltrating endometriosis surgery: a randomized controlled pilot study. J Obstet Gynaecol.
2021;41(2):263-8. Also available: http://dx.doi.org/10.1080/01443615.2020.1755628
. PMID: 32530335.

Material Performance Study - Hyaluronic Acid
|
175
132. Wu X, Hu J, Fan D, Lin X, Wu X, He X, He X, Lan P. Safety and efficacy of sodium hyaluronate gel and chitosan in
preventing postoperative peristomal adhesions after defunctioning enterostomy: a prospective randomized controlled
trials. Medicine (Baltimore). 2015 Dec;94(51):e2354. Also available: http://dx.doi.org/10.1097/MD.0000000000002354
.
PMID: 26705233.
133. Wang YQ, Song XH, Wu SL, Huang YZ, Yan L, Li CZ. Comparison of autocross-linked hyaluronic acid gel and
intrauterine device for preventing intrauterine adhesions in infertile patients: a randomized clinical trial. Gynecol Minim
Invasive Ther. 2020 Apr;9(2):74-80. Also available: http://dx.doi.org/10.4103/GMIT.GMIT_103_19
.
134. Chen J, Wang X, Chen L, Liu J. Influence of hyaluronan nasal dressing on clinical outcome after endoscopic sinus
surgery: a systematic review and meta-analysis. Am J Rhinol Allergy. 2017 Jul;31(4):256-9. Also available:
http://dx.doi.org/10.2500/ajra.2017.31.4438
. PMID: 28639541.
135. Fong E, Garcia M, Woods CM, Ooi E. Hyaluronic acid for post sinus surgery care: systematic review and meta-analysis.
J Laryngol Otol. 2017 Jan;131(S1):S2-S11. Also available: http://dx.doi.org/10.1017/S0022215116009269
. PMID:
28164779.
136. Deng R, Fang Y, Shen J, Ou X, Liuyan W, Wan B, Yuan Y, Cheng X, Shu Y, Chen B. Effect of esterified hyaluronic acid
as middle ear packing in tympanoplasty for adhesive otitis media. Acta Oto-Laryngologica. 2018 Feb;138(2):105-9. Also
available: http://dx.doi.org/10.1080/00016489.2017.1384057. PMID: 29073815.
137. Shi R, Zhou J, Wang B, Wu Q, Shen Y, Wang P, Wang J, Wang Y, Chen Y, Shu XZ. The clinical outcomes of new
hyaluronan nasal dressing: a prospective, randomized, controlled study. Am J Rhinol Allergy. 2013 Jan-Feb;27(1):71-6.
Also available: http://dx.doi.org/10.2500/ajra.2013.27.3833
. PMID: 23406605.
138. Wu W, Cannon PS, Yan W, Tu Y, Selva D, Qu J. Effects of Merogel coverage on wound healing and ostial patency in
endonasal endoscopic dacryocystorhinostomy for primary chronic dacryocystitis. Eye. 2011 Jun;25(6):746-53. Also
available: http://dx.doi.org/10.1038/eye.2011.44
. PMID: 21394118.
139. Al-Shammari NM, Shafshak SM, Ali MS. Effect of 0.8% hyaluronic acid in conventional treatment of moderate to severe
chronic periodontitis. J Contemp Dent Pract. 2018 May;19(5):527-34. Also available:
http://dx.doi.org/10.5005/jp-
journals-10024-2294. PMID: 29807962.
140. Sapna N, Vandana KL. Evaluation of hyaluronan gel (Gengigel(®) ) as a topical applicant in the treatment of gingivitis.
J Investig Clin Dent. 2011 Aug;2(3):162-70. Also available: http://dx.doi.org/10.1111/j.2041-1626.2011.00064.x
. PMID:
25426786.
141. Salih EM, Eldamanhory H, Selmy GI, Galal HA. Comparison of subureteral endoscopic injection of
dextranomer/hyaluronic acid and Lich-Gregoir ureteral reimplantation in the treatment of pediatric primary
vesicoureteral reflux: a prospective randomized study. J Laparoendosc Adv Surg Tech A. 2021 Jun;31(6):719-23. Also
available: http://dx.doi.org/10.1089/lap.2020.0973
. PMID: 33751917.
142. Graf W, Mellgren A, Matzel KE, Hull T, Johansson C, Bernstein M, NASHA Dx Study Group. Efficacy of dextranomer in
stabilised hyaluronic acid for treatment of faecal incontinence: a randomised, sham-controlled trial. Lancet. 2011 Mar
19;377(9770):997-1003. PMID: 21420555.
143. Kirchin V, Page T, Keegan PE, Atiemo KOM, Cody JD, McClinton S, Aluko P. Urethral injection therapy for urinary
incontinence in women. Cochrane Database Syst Rev. 2017 Jul 25;(7):1-67. Also available:
http://dx.doi.org/10.1002/14651858.CD003881.pub4
. PMID: 28738443.
144. De Vita D, Antell H, Giordano S. Effectiveness of intravesical hyaluronic acid with or without chondroitin sulfate for
recurrent bacterial cystitis in adult women: a meta-analysis. Int Urogynecol J Pelvic Floor Dysfunct. 2013
Apr;24(4):545-52. Also available: http://dx.doi.org/10.1007/s00192-012-1957-y
. PMID: 23129247.
145. Özkidik M. Assessment of long-term intravesical hyaluronic acid, chondroitin sulfate and combination therapy for
patients with bladder pain syndrome. Cent European J Urol. 2019;72(3):270-5. Also available:
http://dx.doi.org/10.5173/ceju.2019.0007
. PMID: 31720029.
146. Prada PJ, Cardenal J, García Blanco A, Anchuelo J, Ferri M, Fernández G, Arrojo E, Vázquez A, Pacheco M, Fernández J.
High-dose-rate interstitial brachytherapy as monotherapy in one fraction for the treatment of favorable stage prostate
cancer: toxicity and long-term biochemical results. Radiother Oncol. 2016 Jun;119(3):411-6. Also available:
http://dx.doi.org/10.1016/j.radonc.2016.04.006
.

Material Performance Study - Hyaluronic Acid
|
176
147. Chapet O, Decullier E, Bin S, Faix A, Ruffion A, Jalade P, Fenoglietto P, Udrescu C, Enachescu C, Azria D. Prostate
hypofractionated radiation therapy with injection of hyaluronic acid: acute toxicities in a phase 2 study. Int J Radiat
Oncol Biol Phys. 2015 Mar 15;91(4):730-6. Also available: http://dx.doi.org/10.1016/j.ijrobp.2014.11.027
. PMID:
25752385.
148. Savarino V, Pace F, Scarpignato C, Astegiano M, Calabrese C, Cicala M, Ciliberto E, Conigliaro R, Costamagna G, Cuomo
R, Di Leo A, Di Simone MP, Esposito P, Groppo M, Marchi S, Neri M, Pace F, Savarino V. Randomised clinical trial:
mucosal protection combined with acid suppression in the treatment of non-erosive reflux disease - efficacy of Esoxx, a
hyaluronic acid?chondroitin sulphate based bioadhesive formulation. Aliment Pharmacol Ther. 2017 Mar;45(5):631-42.
Also available: http://dx.doi.org/10.1111/apt.13914
. PMID: 28116754.
149. Wang CC, Wu SH, Tu YK, Lin WJ, Liu SA. Hyaluronic acid injection laryngoplasty for unilateral vocal fold paralysis - a
systematic review and meta-analysis. Cells. 2020 Nov 5;9(11):2417. Also available:
http://dx.doi.org/10.3390/cells9112417
. PMID: 33167303.
150. van der Wal JBC, Iordens GIT, Vrijland WW, van Veen RN, Lange J, Jeekel J. Adhesion prevention during laparotomy:
long-term follow-up of a randomized clinical trial. Ann Surg. 2011 Jun;253(6):1118-21. Also available:
http://dx.doi.org/10.1097/SLA.0b013e318217e99c. PMID: 21502860.
Appendix F. Surveillance Event Reports - PSO and Accident
Investigation
Provided with this report as a separate Excel spreadsheet.

Material Performance Study - Hyaluronic Acid
|
177
Appendix G. Regulatory and Manufacturer Safety Alerts
The associated alerts are provided with this report as a separate PDF.
